Amino-substituted rutaecarpin analog, and synthesis method and application thereof in preparation of anti-obesity medicaments
A technology of evodiamine and its analogues, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as poor water solubility, poor oral absorption, and limited application prospects, and achieve the effect of inhibiting lipogenesis and reducing fat accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
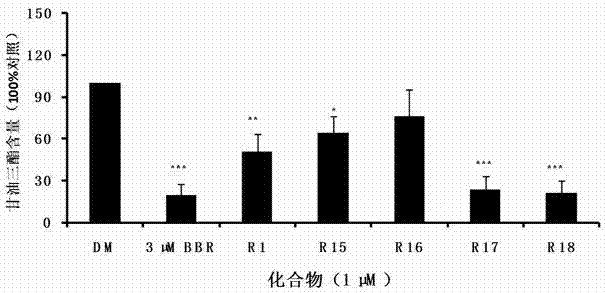
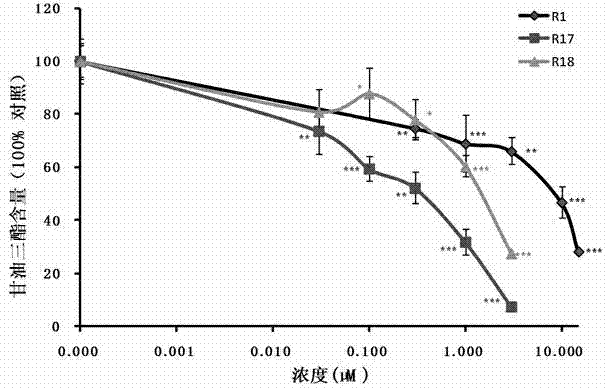
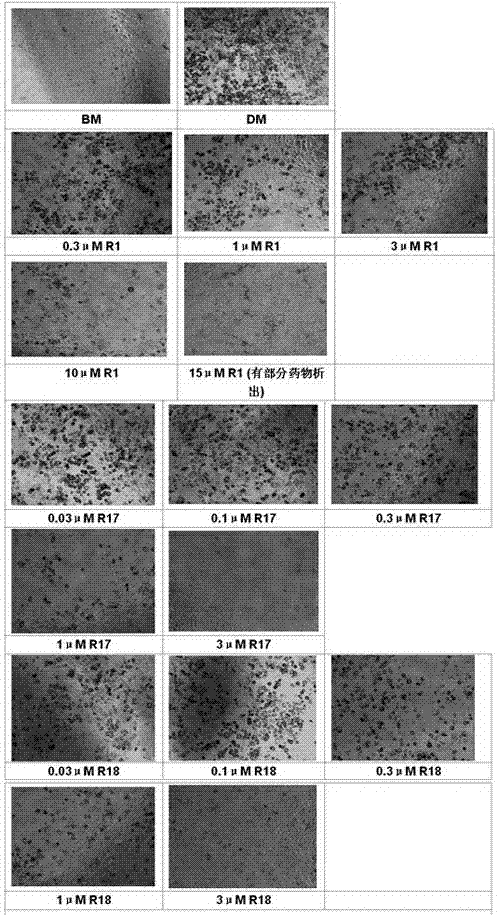
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of compound 2
[0032] Add 0.01mol anthranilamide to 10 ml of triethyl orthopropionate, heat and reflux at 155°C for 12h, then cool to 0°C and let stand for 1-2h, carefully filter the crystals, wash with a small amount of ethanol, and dry in vacuo Afterwards, white needle-like crystals 2 were obtained.
[0033] Yield: 71%; 1 H-NMR (400 MHz, DMSO)δ(ppm): 12.12 (s, 1H), 8.08 (dd, J = 7.9, 1.4, 1H), 7.83–7.73 (m, 1H), 7.60 (d, J = 8.1 , 1H), 7.51–7.41 (m, 1H), 2.62 (q, J = 7.5, 2H), 1.25 (t, J = 7.5, 3H); ESI-MS m / z: 175 [M+H] + .
[0034]
[0035] Compound 2.
Embodiment 2
[0036] Example 2: Synthesis of compound 3
[0037]Dissolve 0.01mol of compound 2 and 0.01mol of sodium acetate in 120ml of acetic acid, heat to 60°C, and slowly add 0.01mol of liquid Br 2 10ml of acetic acid solution. After the dropwise addition, the reaction solution continued to stir until the liquid Br in the reaction solution 2 The brown color disappeared (about 3h), and the reaction solution was poured into 300ml of ice water, resulting in a large amount of white precipitate, which was collected by filtration and vacuum-dried to obtain a white solid 3.
[0038] Yield: 69%; 1 H-NMR (400 MHz, DMSO)δ(ppm): 12.46 (s, 1H), 8.16 – 8.09 (m, 1H), 7.87–7.80 (m, 1H), 7.69 (d, J = 8.1, 1H), 7.55 (t, J = 7.5, 1H), 5.10 (q, J = 6.8, 1H), 2.01 (d, J = 6.8, 3H); ESI-MS m / z: 253 [M+H] + .
[0039]
[0040] Compound 3.
Embodiment 3
[0041] Embodiment 3: Synthesis of Compound 4
[0042] Dissolve 0.01 mol of compound 3 and 0.04 mol of phenylhydrazine in 80 ml of ethanol, heat and reflux for 10 h, after cooling the reaction solution produces a large amount of orange cotton-like solid, filter the precipitate, wash with a small amount of ethanol and dry, then recrystallize with ethanol An orange solid 4 was obtained.
[0043] Yield: 82%; 1 H-NMR (400 MHz, DMSO) δ (ppm): 1H NMR (400 MHz, DMSO) δ 11.46 (s, 1H), 9.86 (s, 1H), 8.13 (dd, J = 7.9, 1.1, 1H), 7.85–7.76 (m, 1H), 7.67 (d, J = 8.1, 1H), 7.58 (d, J = 8.2, 2H), 7.48 (t, J = 7.5, 1H), 7.28 (t, J = 7.8, 2H), 6.89 (t, J = 7.3, 1H), 2.35 (s, 3H); ESI-MS m / z: 279 [M+H] + .
[0044]
[0045] Compound 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com