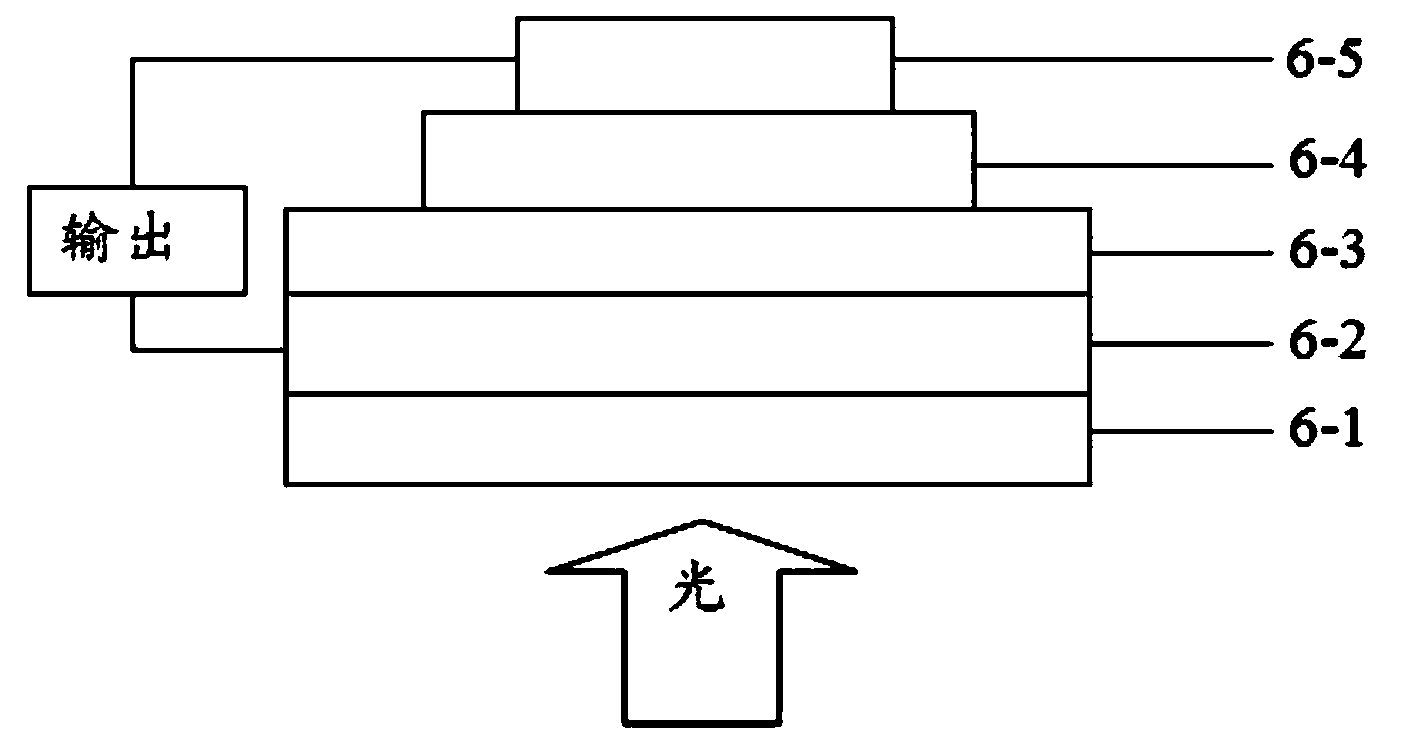
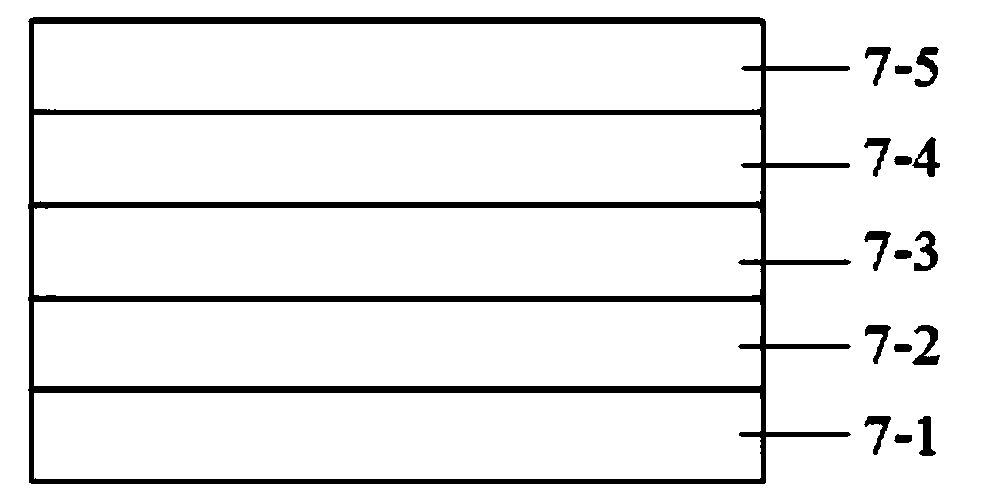
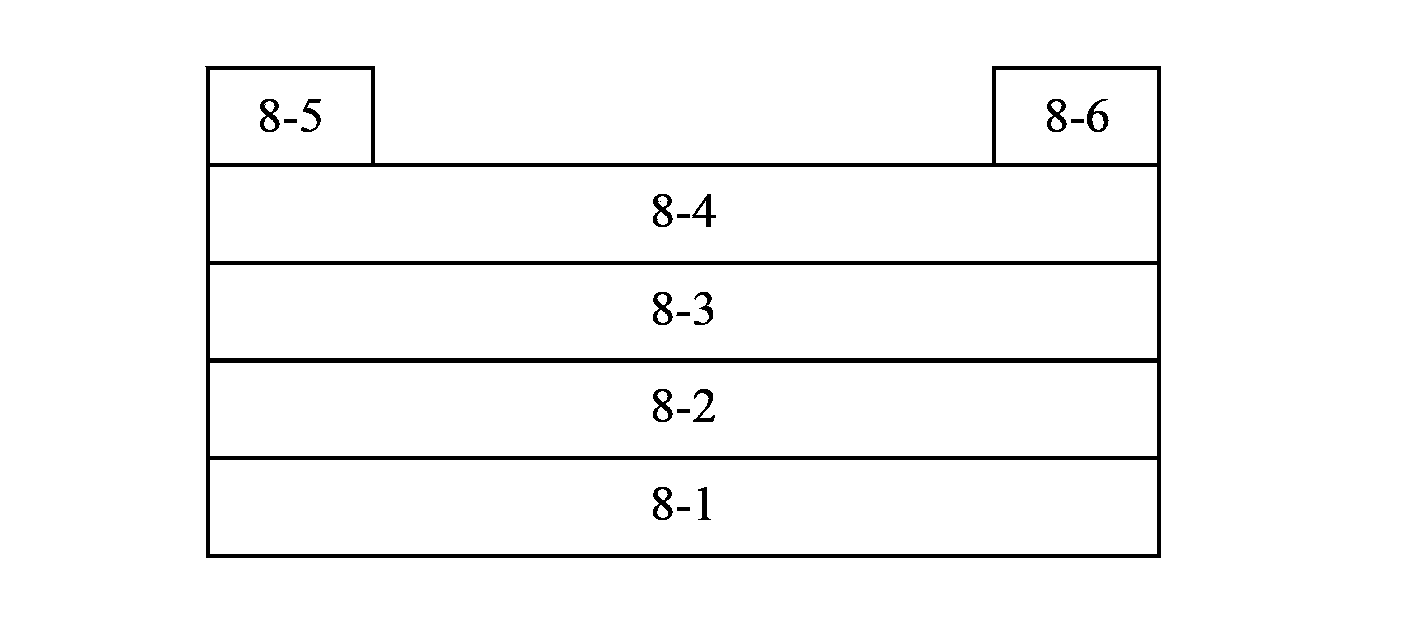
Polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene, preparation method and application thereof
A technology of benzothiadiazole and dithiophene, which is applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, organic chemistry, etc., can solve the problem that the absorption spectrum cannot be well matched with the solar spectrum, the energy transfer efficiency is low, and the problems such as low energy conversion efficiency, to achieve the effect of expanding the light absorption range, improving the carrier mobility, and reducing the band gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene is specifically polymer P1 having the following general formula, where n=5:
[0066] P1:
[0067] The preparation method includes the following steps:
[0068] 1. The preparation method of compound A1 includes the following steps:
[0069]
[0070] (1) Preparation of 2,5-dibromo-spiro[cyclopenta[2,1-b;3,4-b']dithiophene-7,7'-benzofluorene] (d):
[0071] Provide compound a, which is 2-bromophenylnaphthalene; provide compound b, which is 2,6-dibromocyclopenta[2,1-b;3,4-b’]dithiophen-4-one;
[0072] Under the protection of nitrogen, add 1.42g (5mmol) of compound a to 60mL of anhydrous tetrahydrofuran, cool to -78°C, slowly add 3mL of n-butyllithium in n-hexane solution (2.5mol / L, 7.5mmol), and complete the addition. After stirring and reacting at -78°C for 1h, slowly add dropwise the solution obtained by dissolving 1.83g (5mmol) of compound b and 15ml of dry THF, returning to room temperature, and cont...
Embodiment 2
[0090] A polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene is specifically polymer P2 having the following general formula, where n=20:
[0091] P2:
[0092] The preparation method includes the following steps:
[0093] 1. The preparation method of compound A2 includes the following steps:
[0094]
[0095] (1) Preparation of 2,5-dibromo-spiro[cyclopenta[2,1-b;3,4-b']dithiophene-7,7'-benzofluorene] (d):
[0096] Provide compound a, which is 2-bromophenylnaphthalene; provide compound b, which is 2,6-dibromocyclopenta[2,1-b;3,4-b’]dithiophen-4-one;
[0097] Under the protection of nitrogen, add 1.42g (5mmol) of compound a to 60mL of anhydrous tetrahydrofuran, cool to -78°C, slowly add 2.4mL of n-butyllithium in n-hexane solution (2.5mol / L, 6mmol), and complete the addition. After stirring and reacting at -78°C for 1h, slowly add dropwise the solution obtained by dissolving 2.2g (6mmol) of compound b and 20ml of dry THF, returning to room temperature, and cont...
Embodiment 3
[0115] A polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene is specifically polymer P3 having the following general formula, where n=40:
[0116] P3:
[0117] The preparation method includes the following steps:
[0118] 1. The preparation method of compound A3 includes the following steps:
[0119]
[0120] (1) Preparation of 2,5-dibromo-spiro[cyclopenta[2,1-b;3,4-b']dithiophene-7,7'-benzofluorene] (d):
[0121] Provide compound a, which is 2-bromophenylnaphthalene; provide compound b, which is 2,6-dibromocyclopenta[2,1-b;3,4-b’]dithiophen-4-one;
[0122] Under the protection of nitrogen, add 1.42g (5mmol) of compound a to 60mL of anhydrous tetrahydrofuran, cool to -78℃, slowly add 2.8mL of n-butyllithium in n-hexane solution (2.5mol / L, 7mmol), and the addition is complete After stirring and reacting at -78°C for 1h, slowly add dropwise the solution obtained by dissolving 2.0g (5.5mmol) of compound b in 20ml of dry tetrahydrofuran, returning to room tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current density | aaaaa | aaaaa |
| Maximum brightness efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com