Supported spinel compound and preparation and application thereof
A spinel and compound technology, applied in the field of supported spinel compounds and their preparation and application, can solve the problems of high metal oxide reduction temperature, poor cycle performance, and less oxygen vacancies, and achieve sustainable reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
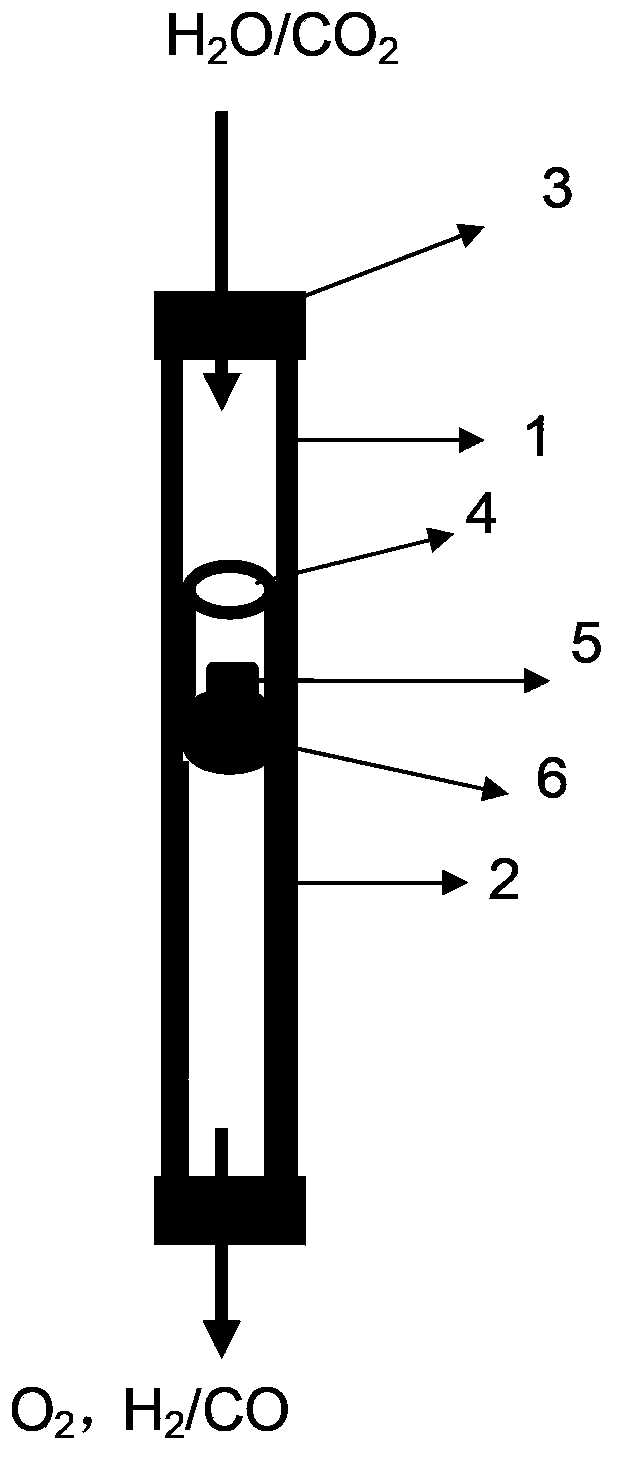
Image
Examples
Embodiment 1
[0046] Preparation of NiFe by Sol-Gel Self-propagating Combustion Method 2 o 4 / SiO 2 : Weigh 2.91g cobalt nitrate Ni (NO 3 ) 2 ·6H 2 O(10mmol), 8.08g Fe(NO 3 ) 3 9H 2 O (20mmol), 9.46g of citric acid (45mmol) were dissolved in 50ml of deionized water, and 19.1mL of silica sol (30%) was added, stirred at room temperature for 30min, placed in a water bath at 80°C until evaporated to dryness, and then Dry in an oven at 120°C until a xerogel is formed. Then, the obtained xerogel was ignited in a muffle furnace at 600 °C and fully burned, and the obtained product was calcined at 700 °C for 4 h to prepare NiFe with a loading capacity of 25%. 2 o 4 / SiO 2 sample.
Embodiment 2
[0048] a: Preparation of Ni by combustion method by sol-gel self-propagating combustion method 0.5 Cu 0.5 Fe 2 o 4 : Weigh 2.91g cobalt nitrate Ni (NO 3 ) 2 ·6H 2 O (10mmol), 2.42g copper nitrate Cu (NO 3 ) 2 ·3H 2 O(10mmol), 16.16g Fe(NO 3 ) 3 9H 2 O (40mmol), 12.60g of citric acid (60mmol) were dissolved in 60ml of deionized water, stirred at room temperature for 30min, placed in an oil bath at 90°C until evaporated to dryness, and dried in an oven at 120°C until dry condensation was formed. glue. Then, the obtained xerogel was ignited in a muffle furnace at 700°C and fully burned, and the obtained product was calcined at 800°C for 3 hours to obtain Ni 0.5 Cu 0.5 Fe2 o 4 .
[0049] b: Mechanical mixing load: Weigh a certain mass of inert high temperature resistant oxide carrier SiO 2 and spinel material Ni 0.5 Cu 0.5 Fe 2 o 4 , so that Ni 0.5 Cu 0.5 Fe 2 o 4 The loading amount of Ni is 30%, ball milled for 8h after mixing evenly, and roasted at 900°C ...
Embodiment 3
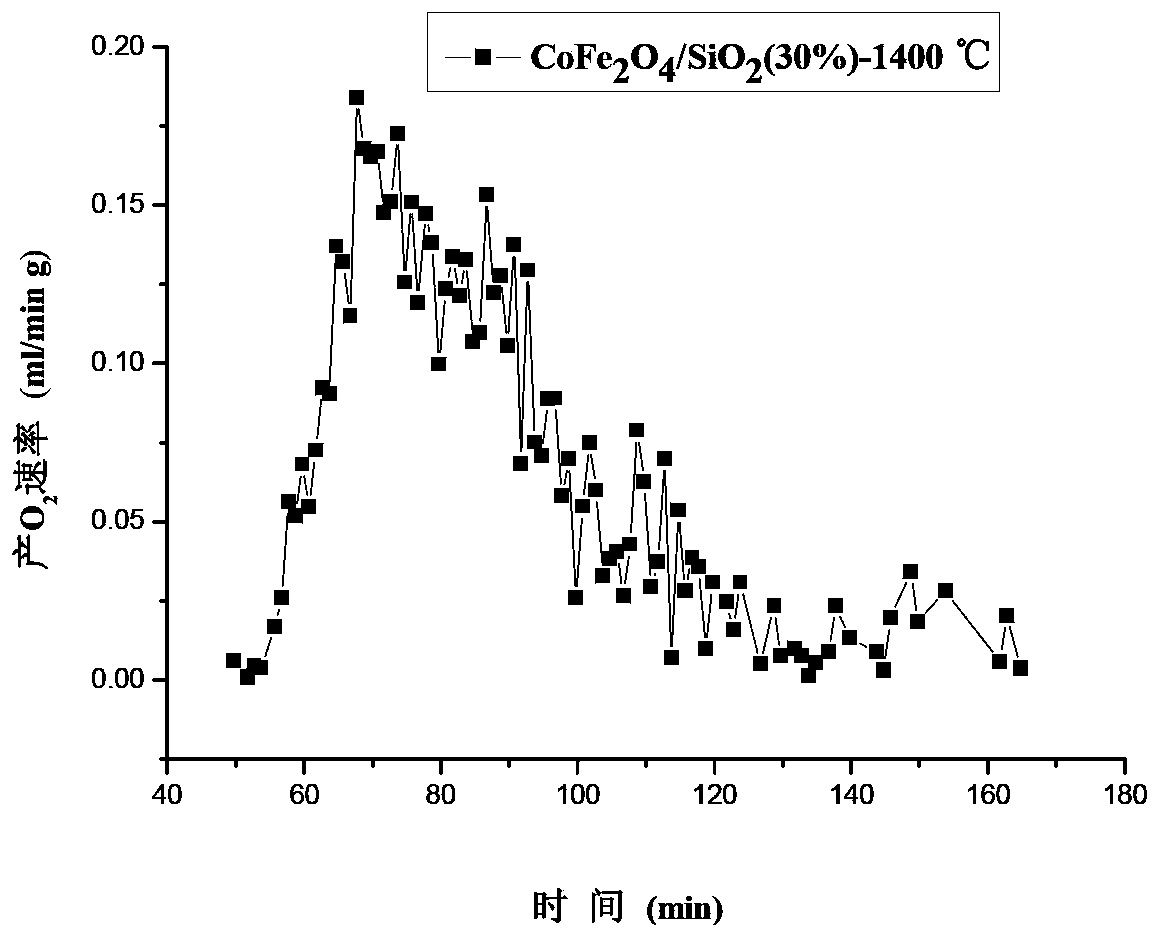
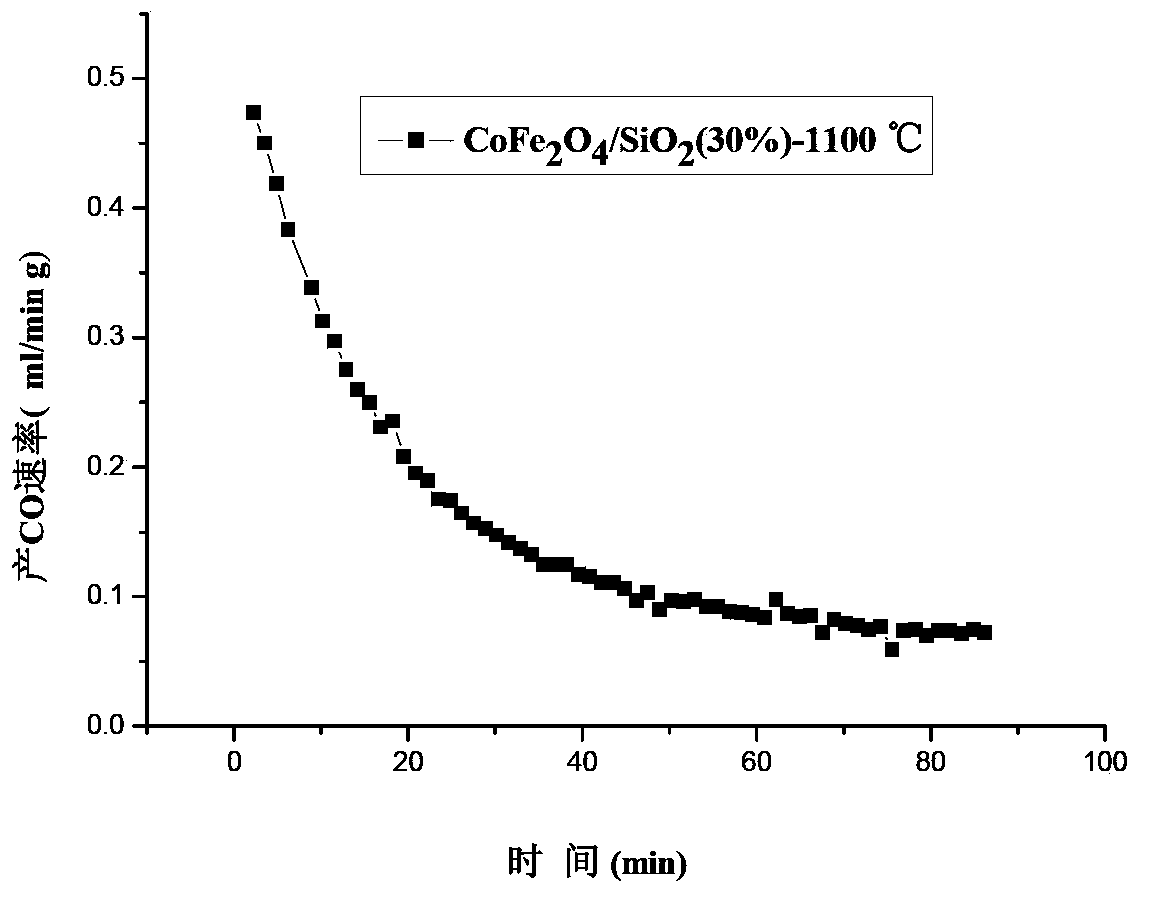
[0051] Weigh 0.400g 25wt%NiFe 2 o 4 / SiO 2 The sample is placed in the reaction tube, the deoxidation temperature is selected as 1400°C, after a period of constant temperature treatment, the temperature is lowered, and CO 2 , the reaction temperature is selected at 1100-1200° C., and the reaction is completed in 2 hours. o 2 Yield and CO production are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com