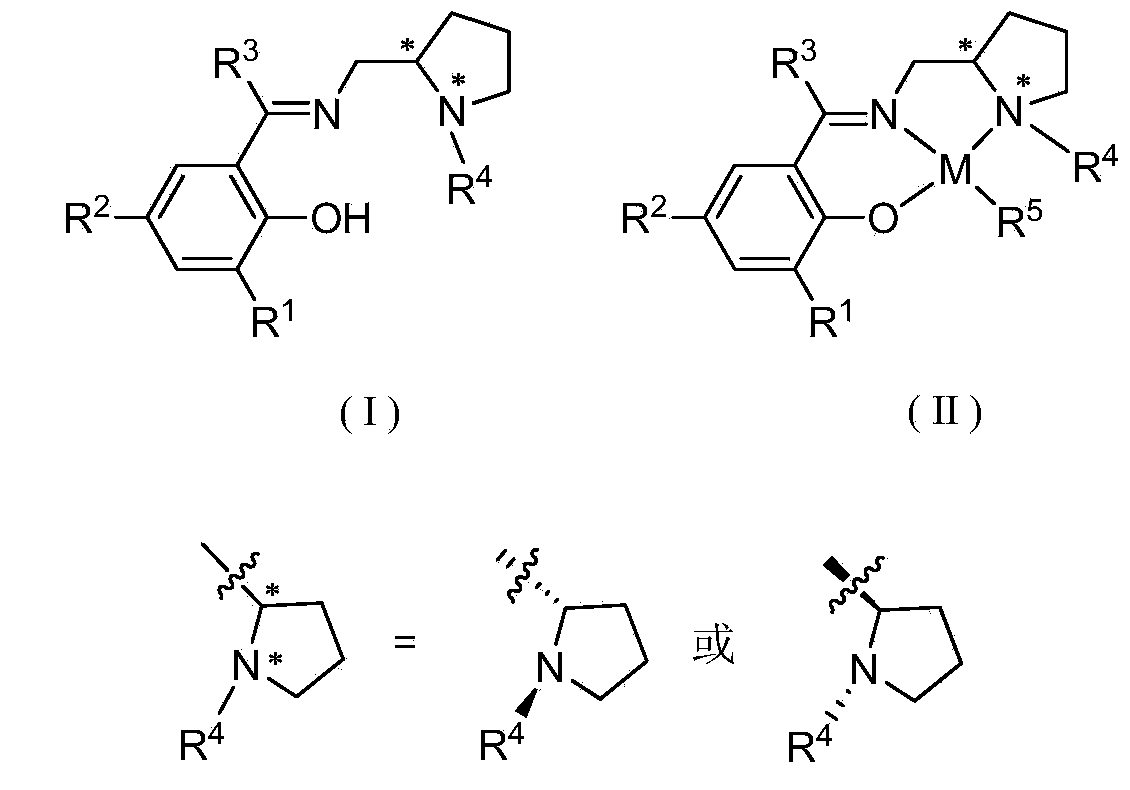
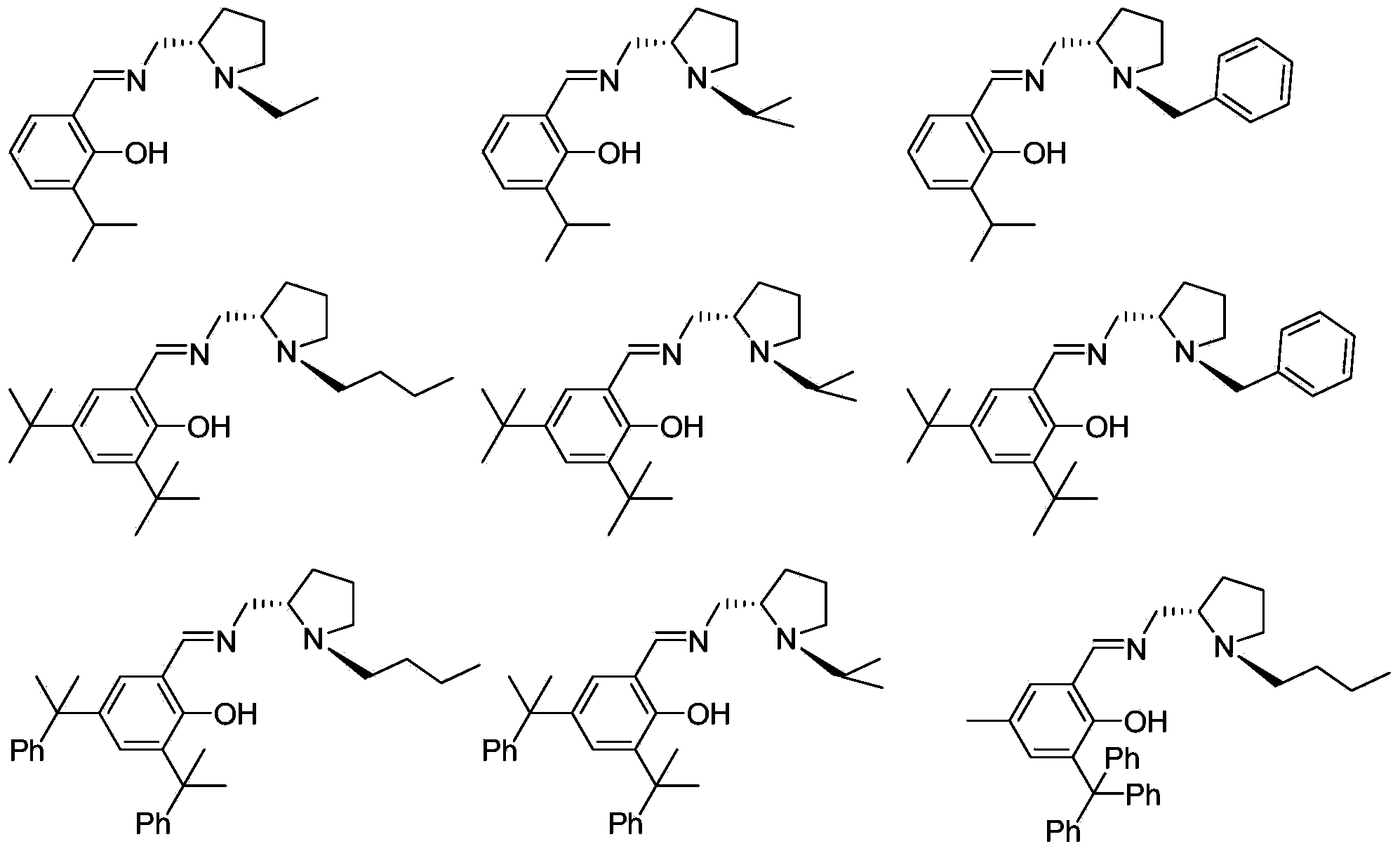
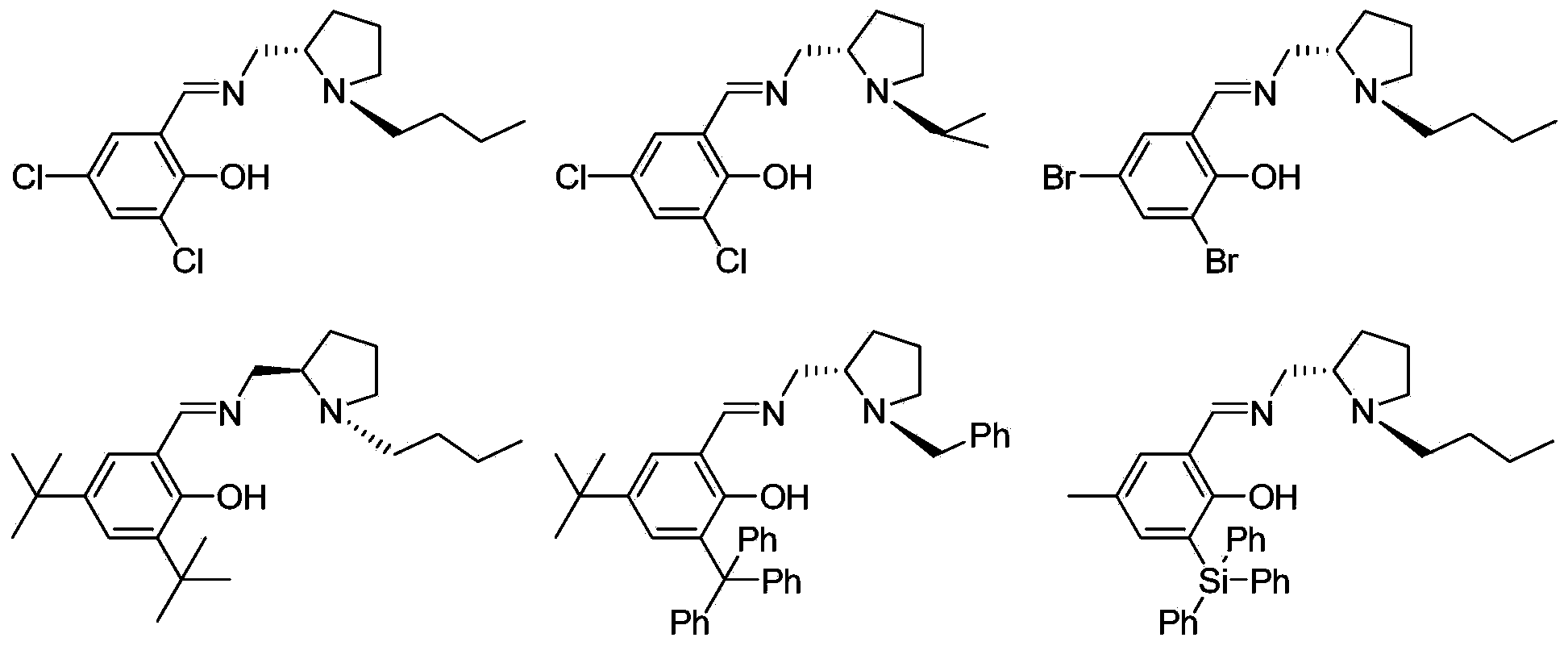
Chiral imine phenol oxyl zinc and magnesium compound as well as preparation method and application thereof
A technology of imine phenoxy zinc and magnesium compounds, which is applied in the direction of magnesium organic compounds, zinc organic compounds, chemical instruments and methods, etc., and can solve the problems of few catalysts and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Ligand L1
[0047] Add 1.65 g of 3-isopropyl salicylaldehyde, 30 mL of absolute ethanol, and 1.35 g of (S)-1-ethyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Dry over anhydrous magnesium sulfate, remove the solvent and excess low-boiling reactants to obtain a yellow viscous liquid. The pure product was obtained without further workup (2.51g, 91.6%).
[0048]
[0049] 1 H NMR (CDCl 3 ,400MHz):δ13.86(s,1H),8.36(s,1H),7.25(d,J=6.4Hz,1H),7.09(d,J=2.4Hz,1H),3.74(ddd,J= 11.8,4.1,0.9Hz,1H),3.36(dd,J=11.8,8.8Hz,1H),3.07(sept,1H),3.01–2.90(m,2H),2.56(dt,J=8.9,6.6Hz ,1H),2.01–1.77(m,2H),1.76–1.69(m,2H),1.45(s,9H),1.31(s,9H),1.15(d,J=6.5Hz,3H),1.05( d,J=6.4Hz,3H). 13 C{ 1 H}NMR (CDCl 3 For C 17 h 26 N 2 O:C,74.41;H,9.55;N,10.21.Found:C,74.54;H,9.69;N,10.15%.
Embodiment 2
[0051] Synthesis of Ligand L2
[0052] Add 2.34g of 3,5-di-tert-butyl salicylaldehyde, 30mL of absolute ethanol, and 1.60g of (S)-1-butyl-2-aminomethyltetrahydropyrrole into a 100mL eggplant-shaped bottle, and heat to reflux for 24 hours . Dry over anhydrous magnesium sulfate, remove the solvent and excess low-boiling raw materials to obtain a yellow viscous liquid (3.58g, 96.1%).
[0053]
[0054] 1 H NMR (CDCl 3,400MHz):δ13.86(s,1H),8.36(s,1H),7.37(d,J=2.4Hz,1H),7.09(d,J=2.4Hz,1H),3.81(dd,J= 11.7,4.6Hz,1H),3.41(dd,J=11.7,7.8Hz,1H),3.21–3.12(m,1H),2.82(ddd,J=11.7,9.5,6.9Hz,1H),2.75–2.65 (m,1H),2.27(ddd,J=11.8,9.2,5.4Hz,0H),2.23–2.17(m,2H),1.96(ddd,J=16.1,12.1,8.2Hz,1H),1.85–1.78 (m,1H),1.78–1.70(m,2H),1.69–1.60(m,1H),1.57–1.46(m,2H),1.44(s,9H),1.31(s,9H),0.90(t ,J=7.3Hz,3H). 13 C{ 1 H}NMR (CDCl 3 For C 24 h 39 N 2 O:C,77.58;H,10.58;N,7.54.Found:C,77.26;H,10.76;N,7.48%.
Embodiment 3
[0056] Synthesis of Ligand L3
[0057] Add 3.58 g of 3,5-dicumyl salicylaldehyde, 30 mL of absolute ethanol, and 1.60 g of (S)-1-butyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Dry over anhydrous magnesium sulfate, remove the solvent and low-boiling raw materials to obtain a yellow viscous liquid (4.71g, 94.8%).
[0058]
[0059] 1 H NMR (CDCl 3 ,400MHz):δ13.45(s,1H),8.21(s,1H),7.31(d,J=2.4Hz,1H),7.28(s,1H),7.27(s,1H),7.26(s, 1H),7.23(s,1H),7.21(s,1H),7.19(d,J=4.6Hz,3H),7.16(d,J=1.9Hz,1H),7.10(d,J=6.8Hz, 1H),7.00(d,J=2.3Hz,1H),3.64(dd,J=11.7,4.7Hz,1H),3.27(dd,J=11.7,7.7Hz,1H),3.09(td,J=6.5 ,3.1Hz,1H),2.73–2.64(m,1H),2.63–2.53(m,1H),2.22–2.10(m,2H),1.82(dd,J=12.3,8.1Hz,1H),1.69( s,6H),1.66(s,3H),1.64(s,3H),1.55–1.44(m,2H),1.40(ddd,J=10.0,7.6,3.6Hz,2H),1.30–1.24(m, 2H),0.85(t,J=7.3Hz,3H). 13 C{ 1 H}NMR (CDCl 3 ,100MHz):165.9,157.9,150.8,150.7,139.3,136.0,128.8,128.0,127.7,126.7,125.6,125.0,118.0,64.4,64.4,55....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com