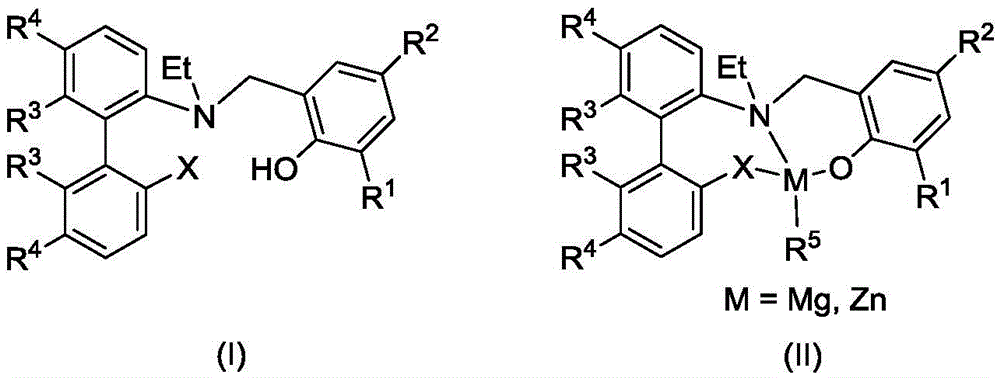
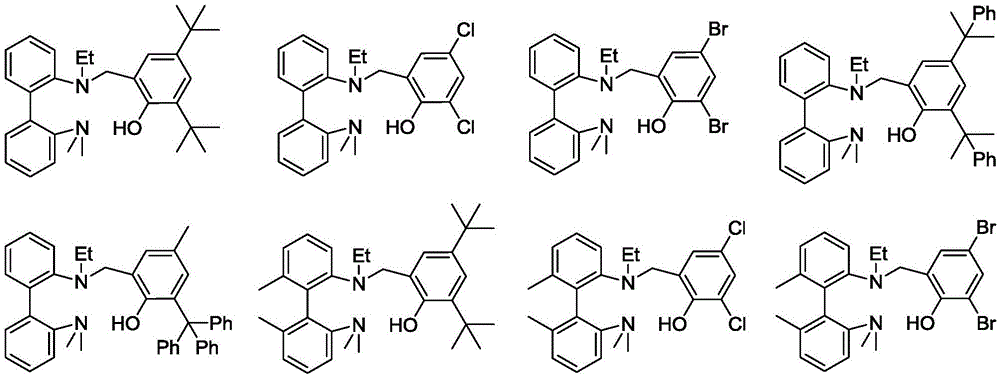
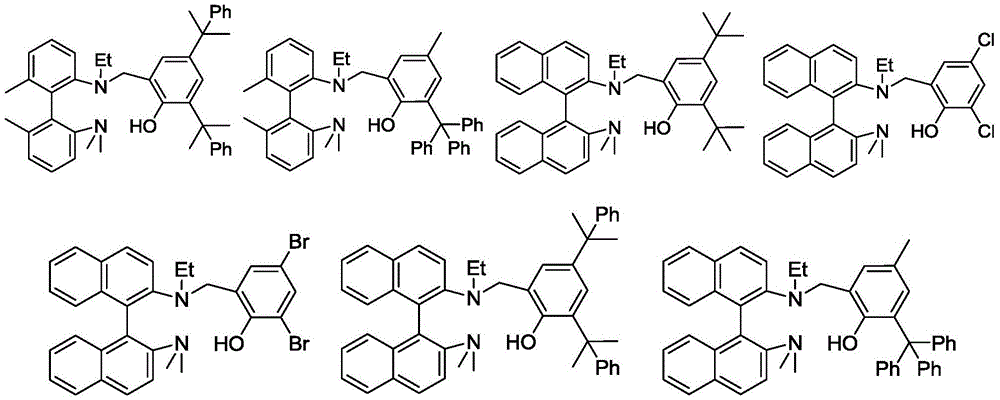
A kind of aminophenol ligand magnesium, zinc complex and its preparation method and application
A technology of aminophenols and complexes, applied in the application field of lactone ring-opening polymerization, can solve the problem of low catalytic activity, achieve high catalytic activity, stable properties, and simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of Ligand L1
[0043] Into a 500mL three-neck flask, add NH 4 Cl (7.865g, 147mmol), 30mLH 2 O, 300mL ethanol, Zn powder (19.160g, 294mmol), 2-nitro-2'-(N,N-dimethylamino)biphenyl (11.845g, 48.9mmol), heated to reflux under stirring. Suction filtration, the filtrate was collected, and the ethanol was removed by rotary evaporation, then 100 mL of water was added to the residue, and the 2 Cl 2 Multiple extractions, organic phase with anhydrous Na 2 SO 4 After drying, filtering, and removing the solvent, the target product 2-amino-2'-(N,N-dimethylamino)biphenyl was obtained as 10.281 g of a colorless liquid with a yield of 99%. 1 HNMR (400MHz, CDCl 3 ):δ7.33(td,J=8.6,J=1.7Hz,1H,Ar-H),7.23–7.16(m,3H,Ar-H),7.12–7.03(m,2H,Ar-H), 6.92(t, J=7.5Hz, 1H, Ar-H), 6.82(d, J=7.9Hz, 1H, Ar-H), 2.64(s, 6H, -N(CH 3 ) 2 ).
[0044] To a 500mL single-necked flask, add glacial acetic acid (43mL, 484mmol), 200mL CH 2 Cl 2 , 2-amino-2'-(N,N-dimethylamino)-biphenyl (10.281...
Embodiment 2
[0050] Synthesis of Ligand L2
[0051] Add 2-(N-ethylamino)-2'-(N,N-dimethylamino)biphenyl (0.961g, 4.0mmol) and 15mL THF to a 100mL single-necked flask, stir at room temperature, add dropwise 20mL of 2 , 4-Dichloro-6-bromomethylphenol (1.54g, 4.0mmol) in THF was added dropwise with Et 3 N (0.84mL, 6.0mmol), reacted for 1.5h, separated by column to obtain 1.06g of white solid, yield 64.1%.
[0052]
[0053] 1 HNMR (400MHz, CDCl 3 ):δ7.43–7.25(m,5H),7.17(d,J=2.3Hz,1H,Ar-H),7.12–7.00(m,3H),6.85(d,J=2.2Hz,1H,Ar -H), 4.34(d, J=14.1Hz, 1H, Ar-CH 2 ), 4.03 (d, J=14.2Hz, 1H, Ar-CH 2 ),2.84–2.75(m,1H,-CH 2 CH 3 ),2.74–2.65(m,1H,-CH 2 CH 3 ),2.45(s,6H,-N(CH 3 ) 2 ),0.75(t,J=7.1Hz,3H,-CH 2 CH 3 ). 13 CNMR (CDCl 3 ,100MHz):δ152.04,151.13,147.14,139.80,133.32,132.42,131.53,128.59,128.22,128.16,126.73,126.67,125.78,124.16,122.83,122.22,121.70,121.16,118.50(AllAr-C),58.93(Ar -CH 2 ),47.46(-CH 2 CH 3 ),43.53(-N(CH 3 ) 2 ),43.50(-N(CH 3 ) 2 ),11.30(-CH 2 CH 3 ).Ana...
Embodiment 3
[0055] Synthesis of Ligand L3
[0056] Add 2-(N-ethylamino)-2'-(N,N-dimethylamino)biphenyl (0.961g, 4.0mmol) and 15mL THF to a 100mL single-necked flask, stir at room temperature, add dropwise 20mL of 2 , 4-dibromo-6-bromomethylphenol (1.37g, 4.0mmol) in THF was added dropwise with Et 3 N (0.84mL, 6.0mmol), reacted for 1.5h, separated by column to obtain 1.35g of white solid, yield 53.5%.
[0057]
[0058] 1 HNMR (300MHz, CDCl 3 ):δ7.46(d,J=2.0Hz,1H,Ar-H),7.34(m,5H,Ar-H),7.11–7.00(m,4H,Ar-H),4.33(d,J= 14.1Hz, 1H, Ar-CH 2 ), 4.02 (d, J=14.1Hz, 1H, Ar-CH 2 ),2.85-2.75(m,1H,-CH 2 CH 3 ),2.73-2.64(m,1H,-CH 2 CH 3 ),2.45(s,6H,-N(CH 3 ) 2 ),0.75(t,J=7.1Hz,3H,-CH 2 CH 3 ). 13 CNMR (CDCl 3 ,100MHz):δ153.43,151.05,146.98,139.66,133.63,133.58,133.20,132.41,131.51,130.21,130.15,128.54,128.11,125.71,124.53,122.14,121.62,118.42,110.66,110.01(AllAr-C),58.72 (Ar-CH 2 ),47.37(-CH 2 CH 3 ),43.50(-N(CH 3 ) 2 ),43.47(-N(CH 3 ) 2 ),11.19(-CH 2 CH 3 ).Anal.Calcd.ForC 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com