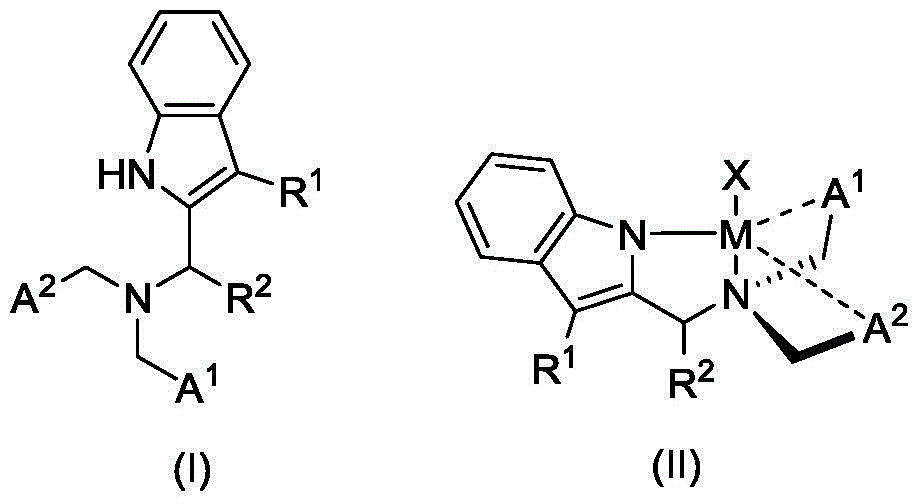
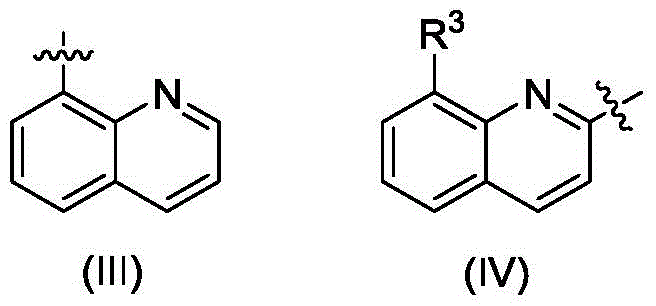
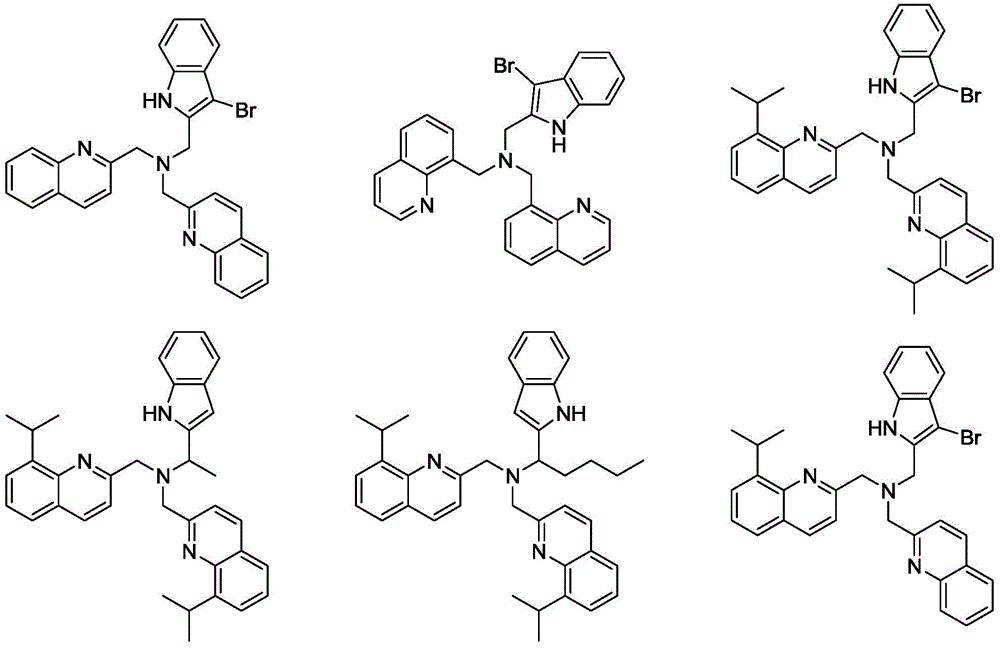
Quinoline amino indolyl zinc, magnesium, calcium compound and its preparation method and application
A kind of aminoindolylzinc, aminoindole technology, applied in calcium compound, quinoline substituted aminoindolylzinc, magnesium field, can solve no isotactic selectivity, highly sensitive, electropositive Strong and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of Ligand L1:
[0056] (1) Synthesis of (3-bromo-1H-indole-2-)methanamine
[0057] 1) Synthesis of N-Boc-3-bromo-2-bromomethylindole
[0058] (method one)
[0059]
[0060] Dissolve N-Boc-2-methylindole (7.51 g, 32.5 mmol), bromosuccinimide (11.56 g, 65.0 mmol) and benzoyl peroxide (0.079 g, 0.325 mmol) in 50 mL of carbon tetrachloride mmol), refluxed at 85°C for 12h, stopped heating, stood to cool, filtered to remove succinimide, concentrated the filtrate, and recrystallized to obtain light yellow needle crystals (9.80g, 77.5%). 1 H NMR (400MHz, CDCl 3 ):δ8.19(d,1H, 3 J=8.4Hz),7.53(d,1H, 3 J=7.8Hz),7.40(dd,1H, 3 J=11.6, 4 J=4.2Hz),7.32(t,1H, 3 J=7.6Hz), 5.07(s, 2H), 1.74(s, 9H).
[0061] (Method Two)
[0062] Carbon tetrachloride dissolved N-Boc-2-methyl-3-bromoindole (3.10g, 10.0mmol), bromosuccinimide (1.78g, 10mmol) and benzoyl peroxide (0.04g, 0.168mmol), after reflux at 85°C for 12h, stop heating, stand for cooling, remove succinimide by fi...
Embodiment 2
[0073] Synthesis of Ligand L2
[0074]
[0075] Except that 2-bromomethylquinoline (6.21g, 28.0mmol) and (3-bromo-1H-indolyl-2-)methanamine (3.15g, 14.0mmol) were used as raw materials, other operating steps were the same as in Example 1 . Recrystallization gave pale yellow crystals (2.00 g, yield 28.2%). 1 H NMR (400MHz, CDCl 3 ):δ11.75(s,1H),8.18(d,2H, 3 J=8.4Hz), 8.11(dd,2H, 3 J=8.4Hz, 4 J=1.6Hz),7.80(d,2H, 3 J=8.0Hz),7.77-7.73(m,2H),7.57-7.53(m,6H),7.24(td,1H, 3 J=8.0Hz, 4 J=1.2Hz),7.18(td,1H, 3 J=7.2Hz, 4 J=0.8Hz), 4.10(s,4H), 4.00(s,2H). 13 C NMR (100MHz, CDCl 3 ): δ159.5, 147.5, 136.9, 135.2, 133.0, 129.8, 128.9, 127.8, 127.6, 127.5, 126.60, 122.5, 121.9, 120.1, 118.9, 111.5, 91.2, 60.2, 48.8. Anal. Calcd. for C 29 h 23 BrN 4 : C, 68.64; H, 4.57; N, 11.04; Found: C, 68.38; H, 4.61; N, 11.07%.
Embodiment 3
[0077] Synthesis of Ligand L3
[0078] (1) Synthesis of 2-bromomethyl-8-isopropylquinoline
[0079]
[0080]Sodium borohydride (3.61g, 95.4mmol) was added batchwise to the ethanol solution of 8-isopropylquinoline-2-carbaldehyde (9.50g, 47.7mmol), stirred at 50°C for 5h, filtered, and the ethanol was spun off, 50mL of dichloro Methane was dissolved, washed with water, separated, dried, filtered, and the solvent was spun off to obtain an orange-yellow liquid (8-isopropylquinoline-2-methanol). In an ice-water bath, methanesulfonyl chloride (6.49g, 56.6mmol) was added dropwise to a dichloromethane solution of the crude product of the previous step, triethylamine (7.16g, 70.8mmol). Stir at room temperature for 2 hours, add 100 mL of water, separate the layers, wash with saturated brine, dry, filter, and spin off the solvent to obtain a brown liquid ([(8-isopropylquinoline-2-)methyl]methanesulfonate). Dissolve the crude product of the previous step and lithium bromide (20.0 g, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com