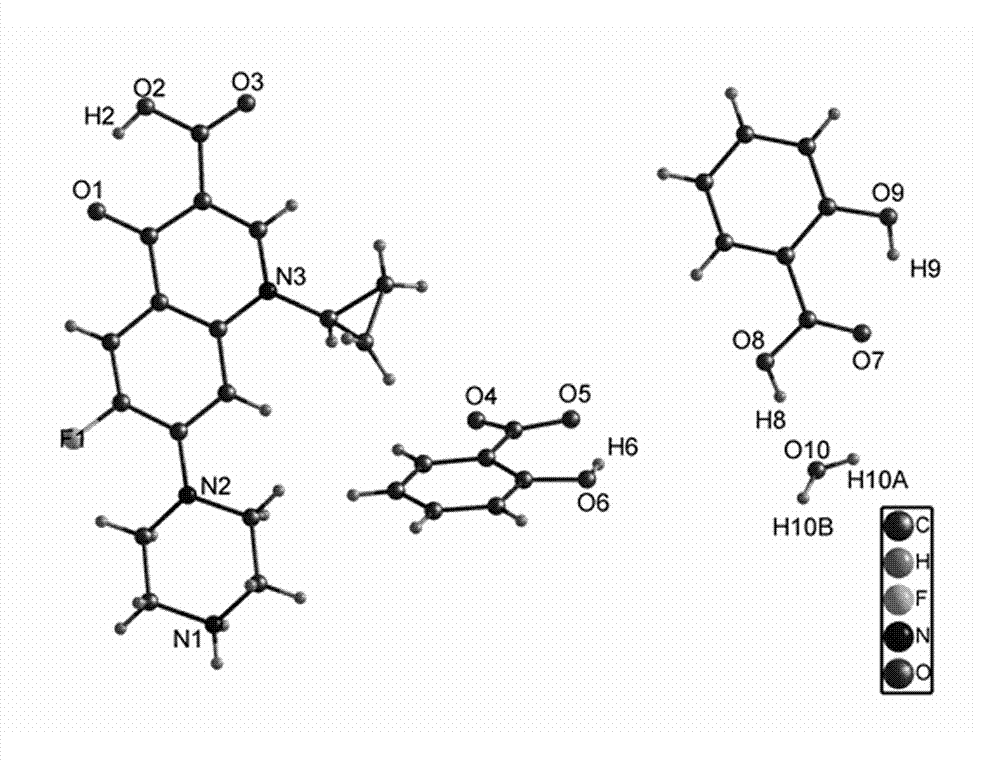
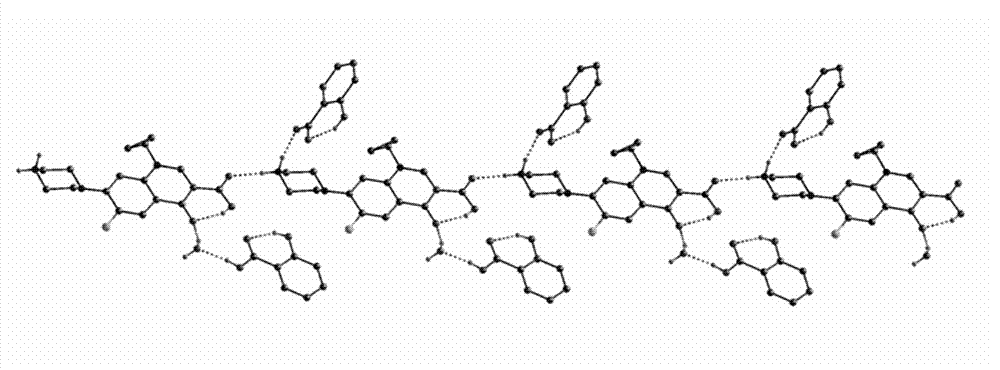
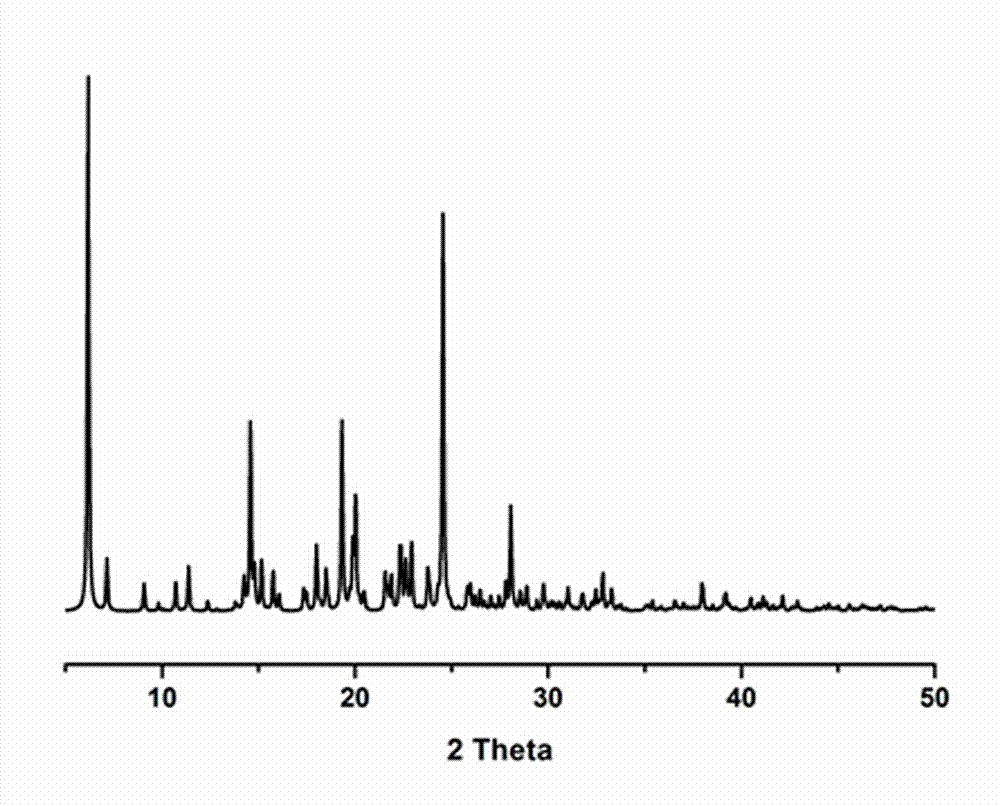
Medicine eutectic of ciprofloxacin and salicylic acid and preparation process thereof
A technology of ciprofloxacin and salicylic acid, which is applied in the field of drug co-crystals, can solve the problems of lack of double synergy, broad-spectrum antibacterial, and narrow application range of mandelic acid, so as to improve drug efficacy and bioavailability, High yield and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of drug co-crystal of ciprofloxacin and salicylic acid
[0029] 0.1 mmol ciprofloxacin (33.1 mg) and 0.1 mmol salicylic acid (13.8 mg) were added to an aqueous methanol solution composed of 6 ml methanol and 3 mL water. After mixing, stir for 15 minutes, let stand for 30 minutes, filter, put the filtrate into a 25ml test tube, seal the hole with plastic wrap to obtain a clear solution and place it at room temperature to volatilize naturally. A solid containing colorless block crystals is obtained after 1 week. The resulting solid-liquid mixture was filtered to collect colorless massive crystals, and dried at 60~70℃ for 12 hours to obtain 33.84mg of drug co-crystal of ciprofloxacin and salicylic acid, with a molar yield of 72% .
Embodiment 2
[0030] Example 2 Preparation of drug co-crystal of ciprofloxacin and salicylic acid
[0031] 0.1 mmol ciprofloxacin (33.1 mg) and 0.1 mmol salicylic acid (13.8 mg) were added to an aqueous methanol solution composed of 6 ml methanol and 3 mL water. After mixing, stir for 15 minutes, let stand for 30 minutes, filter, put the filtrate into a 25ml test tube, seal the hole with plastic wrap to obtain a clear solution and place it at room temperature to volatilize naturally. After 2 weeks, a solid containing colorless block crystals is obtained. The resulting solid-liquid mixture was filtered to collect colorless massive crystals, and dried at 60~70℃ for 12 hours to obtain 35.25mg of drug co-crystal of ciprofloxacin and salicylic acid, with a molar yield of 75% .
Embodiment 3
[0032] Example 3 Preparation of drug co-crystal of ciprofloxacin and salicylic acid
[0033] 0.1 mmol ciprofloxacin (33.1 mg) and 0.1 mmol salicylic acid (13.8 mg) were added to an aqueous methanol solution composed of 6 ml methanol and 3 mL water. After mixing, stir for 15 minutes, let stand for 30 minutes, filter, put the filtrate into a 25ml test tube, seal the hole with plastic wrap to obtain a clear solution and place it at room temperature to volatilize naturally. After 3 weeks, a solid containing colorless block crystals is obtained. The resulting solid-liquid mixture was filtered to collect colorless massive crystals, and dried at 60~70℃ for 12 hours to obtain 37.13mg of drug co-crystal of ciprofloxacin and salicylic acid, with a molar yield of 79% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com