Fluorescent carbon dots, and fused salt preparation method and application thereof
A technology of fluorescent carbon dots and carbon dots, which is applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of complex preparation process, low yield, and poor controllability of carbon dot size, and achieve simple equipment and extensive carbon sources. , the effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Weighing of molten salt: select sodium nitrate-potassium nitrate-sodium nitrite triple molten salt system, and weigh in a mass ratio of 7:53:40;
[0034] (2) Weigh sucrose according to 6% of the total mass in (1);
[0035] (3) Fully mix (1) sodium nitrate, potassium nitrate and sodium nitrite with sucrose carbon source to obtain 5 g of the mixture, put it in a crucible, put it into a heating furnace, and heat at 180 ° C for 8 h.
[0036] (4) Take 5g of the heat-treated mixture, add 50ml of deionized water to dissolve it completely, transfer it to a dialysis bag for dialysis, select a molecular weight cut-off of 3500MW, and dialyze for 3 days. The required fluorescent carbon dots are obtained in the solution after centrifugal dialysis, and take the dialysis bag The medium solution is preserved, and the molten salt in the dialysis cup can be recycled and reused.
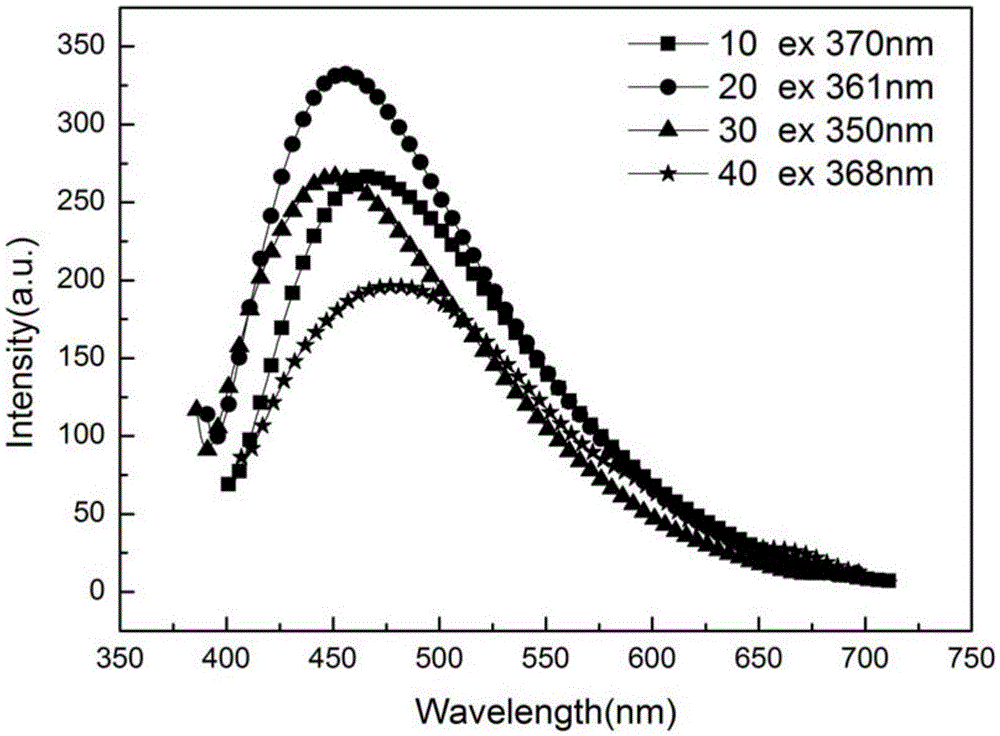
[0037] (5) The solution in the dialysis bag was diluted 10, 20, 30, 40 times with deionized water, and t...
Embodiment 2
[0041] (1) Weighing of molten salt: select potassium nitrate-sodium nitrite molten salt system, and weigh at a mass ratio of 9:10;
[0042] (2) Weighing glucose according to 2% of the total mass in (1);
[0043] (3) (1) Potassium nitrate and sodium nitrite were fully mixed with glucose carbon source to obtain a treated mixture, 5 g of the mixture was placed in a crucible, put into a heating furnace, and heated at 150° C. for 8 hours.
[0044] (4) After heating, add 50ml of deionized water to the crucible to dissolve completely, then transfer to a dialysis bag for dialysis, select a molecular weight cut-off of 3000MW, and dialysis for 3 days, obtain the required fluorescent carbon dots in the solution after centrifugal dialysis, and take the dialysis bag The solution in the bag is preserved, and the molten salt in the dialysis cup can be recycled and reused.
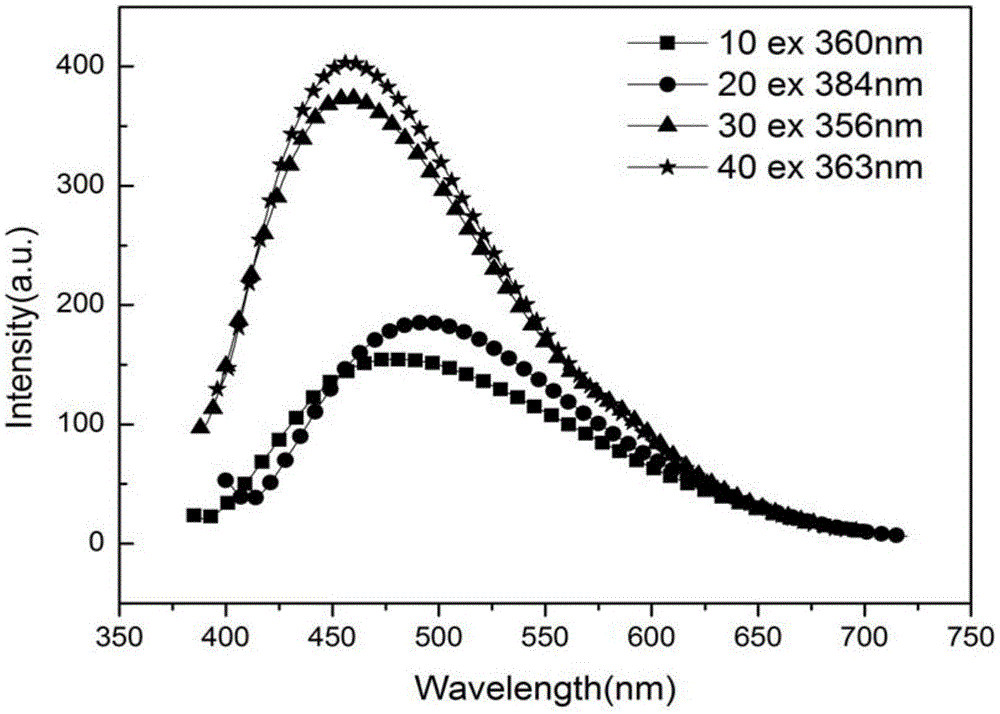
[0045] (5) The solution in the dialysis bag was diluted 10, 20, 30, and 40 times with deionized water, and the charact...
Embodiment 3
[0049] (1) Weighing of molten salt: choose sodium nitrate-potassium nitrite molten salt system, and weigh according to the mass ratio of 1:1;
[0050] (2) Weigh citric acid according to 11% of the total mass in (1);
[0051] (3) Fully mix (1) sodium nitrate and potassium nitrite with citric acid carbon source to obtain the treated mixture, put 5 g of the mixture in a crucible, put it into a heating furnace, and heat at 160°C for 4 hours.
[0052] (4) After heating, add 100ml of deionized water into the crucible to completely dissolve it, then transfer it to a dialysis bag for dialysis, select a molecular weight cut-off of 5000MW, and dialysis for 2 days. The solution in the bag is preserved, and the molten salt in the dialysis cup can be recycled and reused.
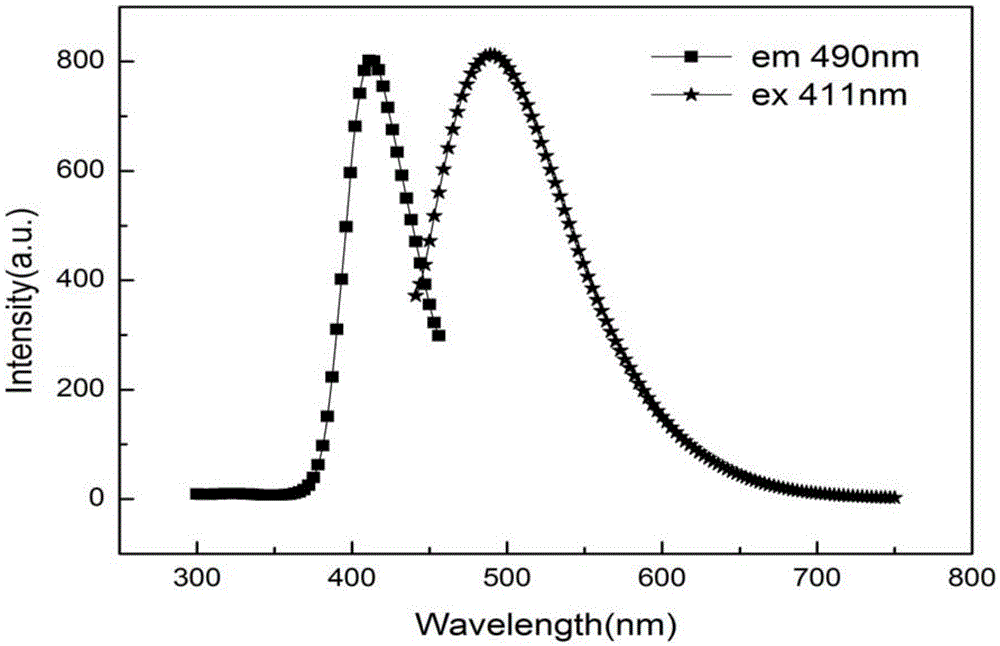
[0053] The prepared carbon dots were characterized by fluorescence using Horiba Fluoromax-4 fluorescence spectrometer, such as Figure 11 shown. The optimum excitation wavelength is at 411nm, and the optimum emission ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com