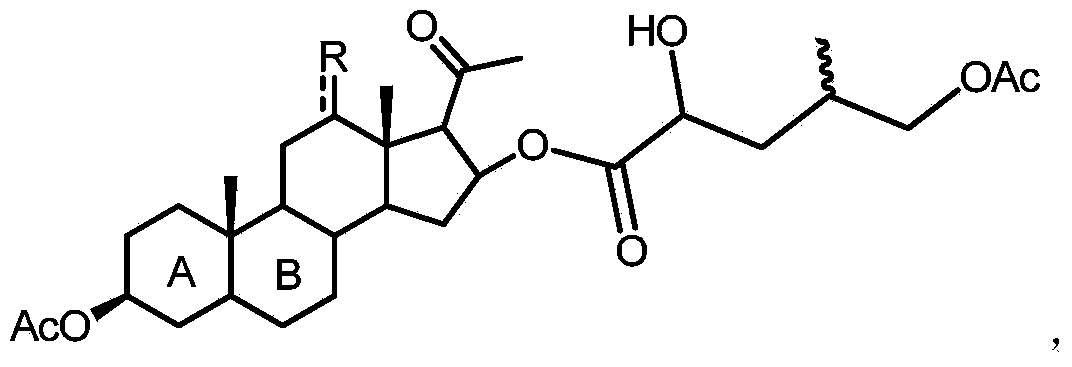
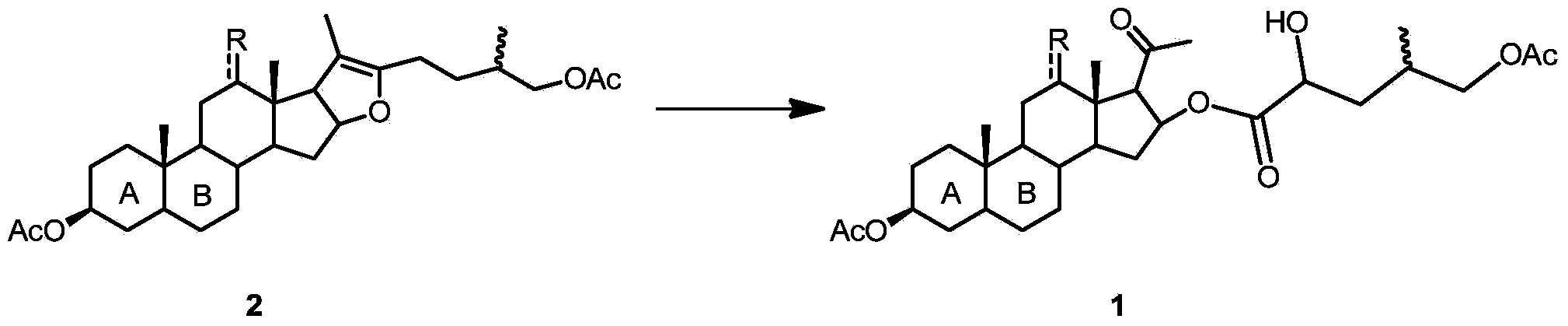
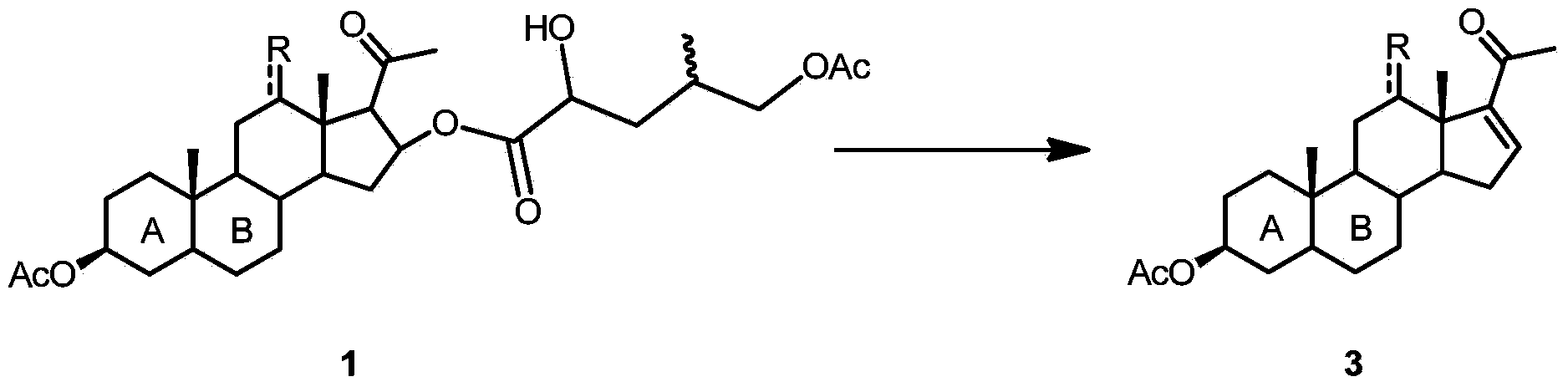
16-(2'-hydroxyl-4'-methyl-5'-acetoxyl) amyl acyloxy acetic acid progesterone alcohol compound as well as synthetic method and application thereof
A technology of valeryloxy acetate pregesterone alcohol and acetoxyl group is applied in the field of 16-valeryloxy acetate pregesterone alcohol compound, and can solve the problems of unstable reaction yield, difficult control of reaction process and the like, and achieves The effect of preventing the hydrolysis reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound 1a
[0029]
[0030] Compound 2a (10 g) and WO 3 (21mg, 0.005e) was dissolved in 60mL of acetone, and when heated to 50°C, 30% hydrogen peroxide (8.1mL, 4eq) was added dropwise, reacted at 50°C for 3 hours, all the raw materials were reacted, quenched with saturated aqueous sodium sulfite solution, and reduced pressure The solvent was evaporated, diluted with 100ml of toluene, washed with water, and concentrated to obtain 10.00g of compound 1a with a yield of 92.1%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ5.59-5.54(m,1H),4.73-4.65(m,1H),4.22-4.18(m,1H),4.12-4.11(m,1H),3.93(d,J=4Hz,2H), 2.10(s,3H),2.08(s,3H),2.05(s,3H),2.02(s,3H),1.03(s,3H),1.01(s,3H),0.98(d,J=4Hz, 3H);
[0032] LRMS-ESI(m / z):629.2([M+Na] + );
Embodiment 2
[0033] The synthesis of embodiment 2 compound 1a
[0034]
[0035] Compound 2a (10g) and Na 2 WO 4 .2H 2 O (24mg, 0.004eq) was dissolved in 60mL butanone, and 30% hydrogen peroxide (6.1mL, 3eq) was added dropwise when heated to 70°C, and reacted at 70°C for 3 hours. The solvent was evaporated under reduced pressure, diluted with 150ml of benzene, washed with water, and concentrated to obtain 10.14g of compound 1a with a yield of 93.4%.
Embodiment 3
[0036] The synthesis of embodiment 3 compound 1b
[0037]
[0038] Compound 2b (10g) and (NH 4 ) 2 MoO 4 (21mg, 0.006eq) was dissolved in 70mL of dichloromethane, 30% hydrogen peroxide (2.0mL, 1eq) was added dropwise at room temperature, and reacted at room temperature for 24 hours. Diluted with methane, washed with water, and concentrated to obtain 10.34 g of compound 1b with a yield of 95.2%.
[0039] 1 H NMR (400MHz, CDCl3 )δ5.59-5.54(m,1H),4.74-4.68(m,1H),4.24-4.20(m,1H),4.12-4.10(m,1H),3.93(d,J=4Hz,2H), 2.11(s,3H),2.08(s,3H),2.06(s,3H),2.02(s,3H),1.03(s,3H),1.01(s,3H),0.99(d,J=4Hz, 3H);
[0040] LRMS-ESI(m / z):629.2([M+Na] + );
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com