Application of heparin oligosaccharide dp12 in atherosclerosis resistance
A technology for atherosclerosis and heparin oligosaccharides, applied in the field of biomedicine, can solve problems such as single structure and biological activity to be studied, and achieve excellent results
Inactive Publication Date: 2014-06-18
CHINA PHARM UNIV
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] The molecular weight of heparin dodecaose is lower than that of low molecular weight heparin, and the structure is also more simple, and its biological activity needs to be studied
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0028] 1) High-fat feed formula
[0029] 6% peanut oil + 2% cholesterol + 92% common feed
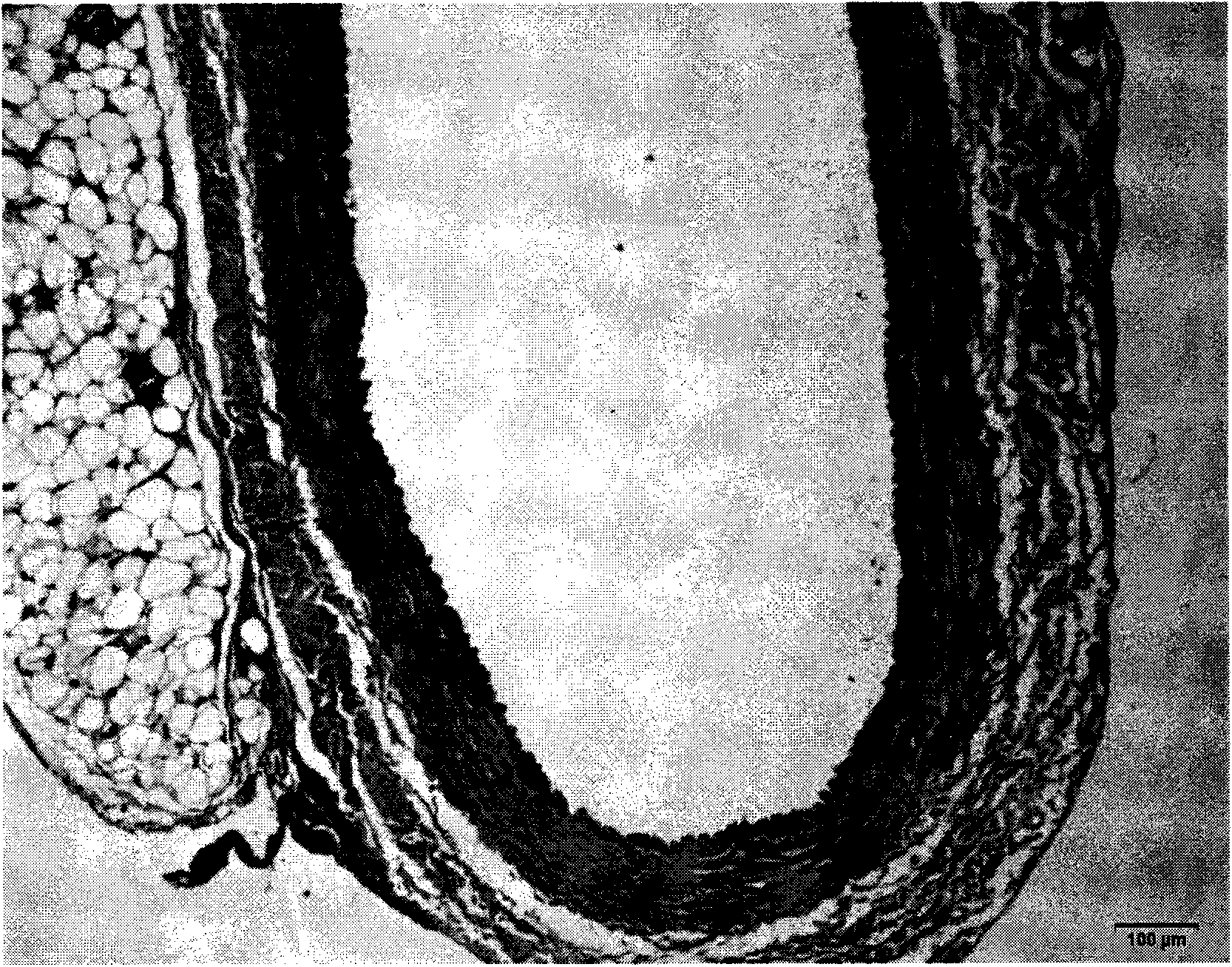
[0030] 2) Establishment of rabbit common carotid artery balloon injury model
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an application of heparin oligosaccharide dp12 in atherosclerosis resistance. According to the application disclosed by the invention, heparin oligosaccharide dp12 is obtained in the laboratory by preliminary study and shown in ZL201010139398.9. In the patent, the atherosclerosis-resistant function of heparin oligosaccharide dp12 is further studied by animal experiments, and the study meets the modern development direction of medicines in China and has important social significance and potential economic benefits in the field of fundamental studies and the field of application studies.
Description
technical field [0001] The invention relates to the field of biomedicine, in particular to heparin dodecaose, which has the effect of resisting atherosclerosis. Background technique [0002] Heparin is a mucopolysaccharide sulfate composed alternately of glucosamine, L-iduroside, N-acetylglucosamine and D-glucuronic acid. It is widely used in clinical anticoagulant and antithrombotic, and is the drug of choice for the prevention and treatment of thromboembolic diseases such as deep venous thrombosis. However, due to its large molecular weight (3000-30000D), uneven biological activity, and side effects such as bleeding, thrombocytopenia, abnormal lipid metabolism and osteoporosis, its clinical application is limited to a certain extent. [0003] Low-molecular-weight heparin is obtained by depolymerization of unfractionated heparin, with a molecular weight of 3000-8000D. Its antithrombotic effect is better than that of heparin, but its anticoagulant effect is lower than that ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08B37/10A61K31/727A61P9/10
Inventor 何书英钱轩王文荟
Owner CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com