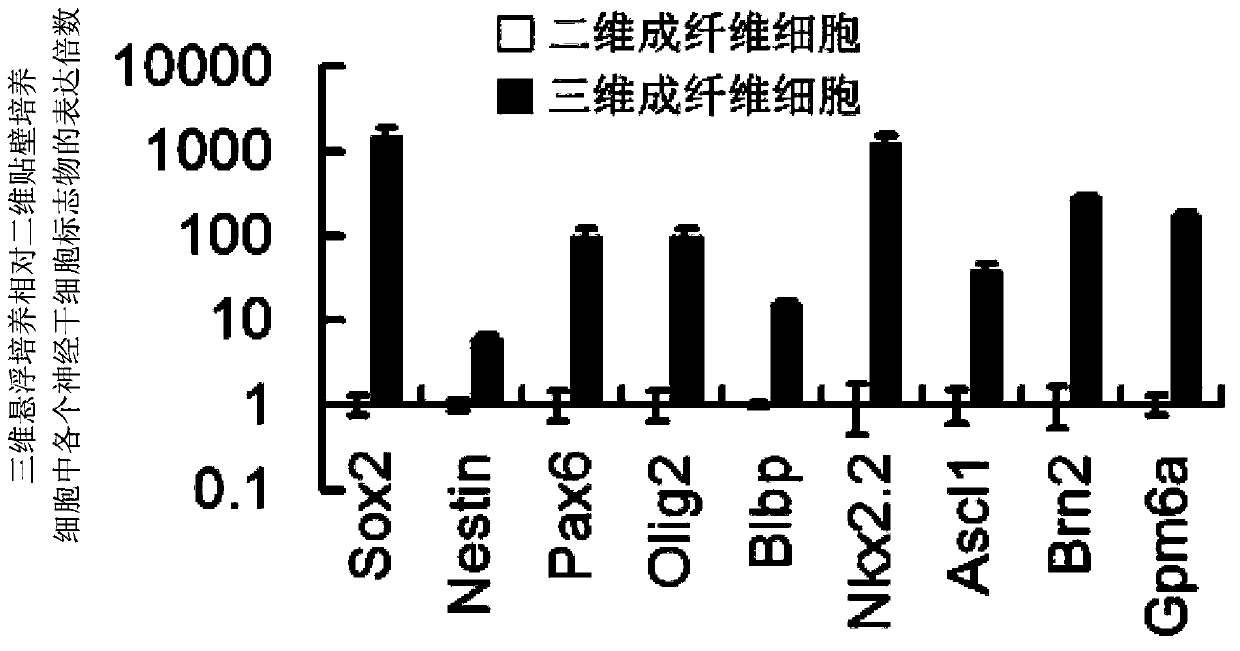
Method for preparing neural stem cells by adopting fibroblasts
A technology of fibroblasts and neural stem cells, applied in animal cells, nervous system cells, vertebrate cells, etc., can solve problems such as cell canceration, and achieve the effect of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, use mouse embryo fibroblast to prepare neural stem cell
[0039] 1. Isolation of mouse embryonic fibroblasts
[0040] Take the 13.5-day pregnant ICR mice that have just died, and after sterilizing with 75% alcohol, cut the abdominal wall to expose the uterine horns. Transfer the uterine horns to a 10cm dish. Wash three times with 10 mL of DPBS buffer. Cut open the embryo sac on each side with scissors and transfer the embryos to a Petri dish. Separate the placenta and membranes from the embryos and remove the head, limbs, viscera, spine, and tail after separation. Transfer the remaining part of the embryo to a new petri dish, wash it three times with 10mL DPBS buffer, cut the tissue into pieces, add 0.25% trypsin-1mM EDTA mixed digestion solution (add 1mL to the remaining tissue fragments of each embryo), Incubate at 37°C for 10 minutes, then stop the trypsin reaction with high-glucose DMEM medium containing 10% (volume ratio) fetal bovine serum, blow a...
Embodiment 2
[0098] Example 2. Using mouse embryonic fibroblasts to prepare neural stem cells and perform differentiation experiments in vivo
[0099] 1. Isolation of mouse embryonic fibroblasts
[0100] GFP transgenic ICR mice were used instead of ICR mice, and the rest were completely the same as Step 1 of Example 1.
[0101] 2. Preparation of Petri dish
[0102] Same as Step 2 of Example 1.
[0103] 3. Culture of mouse embryonic fibroblasts
[0104] Same as Step 3 of Example 1.
[0105] 4. In vivo differentiation experiment
[0106] The cells that completed the three-dimensional suspension culture and the cells that completed the two-dimensional adherent culture in step three were identified as follows:
[0107] 1. Digest the cells with 0.25% trypsin-1mM EDTA mixed digestion solution and resuspend with DPBS buffer to obtain 5×10 4 cells / μl of cell suspension.
[0108]2. Use 10% chloral hydrate to anesthetize adult male SD rats (230–250g) (400mg / kg). Using a stereotaxic instrument...
Embodiment 3
[0116] Embodiment 3, preparation of neural stem cells with mouse tail tip fibroblasts
[0117] 1. Isolation of mouse tail tip fibroblasts
[0118] Take 24-hour-old ICR mice that have just died, after 75% alcohol disinfection, measure from the free end of the tail tip, take 1 / 4-1 / 5 position, cut it with scissors, and inoculate the cut tissue into 10cm Place in a cell culture dish at 37°C, 5% CO 2 When cultured in an incubator, the fibroblasts will gradually climb out of the tissue block, which are P0 generation cells. At 37°C, 5% CO 2 The P0 generation cells were cultured in the incubator, and after the cell confluence reached 95%, the 1:3 passage was carried out. The P1 generation cells were obtained from the first passage, the P2 generation cells were obtained from the second passage, and the P3 generation cells were obtained from the third passage.
[0119] 2. Preparation of Petri dish
[0120] Same as Step 2 of Example 1.
[0121] 3. Culture of mouse tail tip fibroblas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com