Bedside monitoring system
A monitoring system and application system technology, applied in the field of clinical testing, can solve problems such as inability to completely prevent testing, unavoidable batch-to-batch differences, and inability to obtain results, so as to achieve digital management of hospitals, avoid the use of expired reagents, and reduce medical risks. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the embodiments of the present invention. Apparently, the described embodiments are only some of the embodiments of the present invention, not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
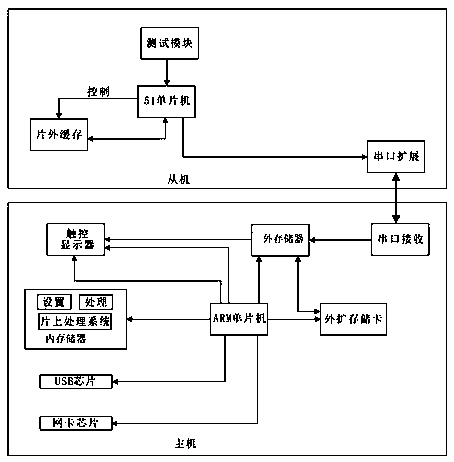
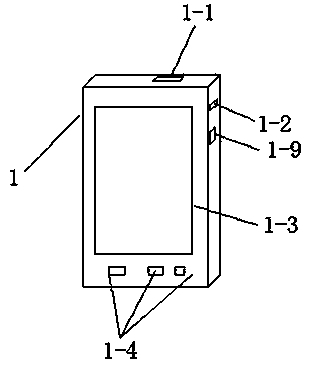
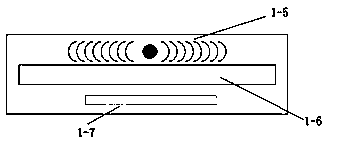
[0048] Such as Figure 1-4 As shown, the bedside monitoring system includes a host 1 for analyzing and processing data and reporting the final result, a slave 2 for collecting data, and a test paper for obtaining raw measurement data in cooperation with the slave 3;
[0049] The host 1 uses the base 4 to communicate with the server system. The host 1 uses a single-chip microcomputer embedded in the operating system, and the corresponding blood glucose application module is i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com