Preparation method of optically pure Lansoprazole
A technology for lansoprazole and enantiomer, which is applied in the field of preparation of optically pure lansoprazole, can solve the problems of long reaction time and the like, and achieves the effects of mild conditions, simple method and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
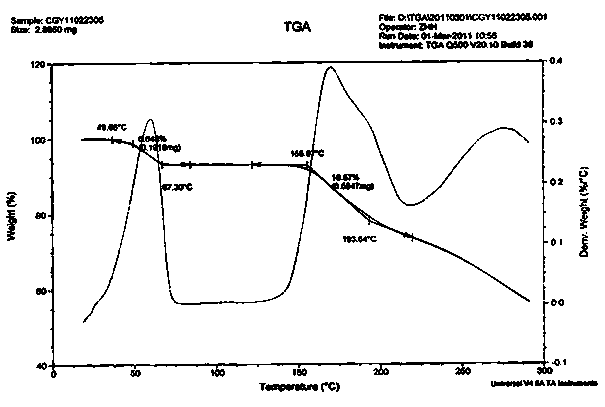
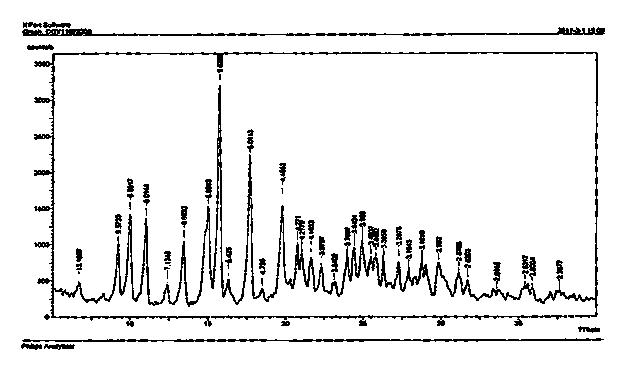
Image
Examples
Embodiment
[0061] The present invention will be further described in detail below through specific examples. However, it should not be understood that the scope of the above subject matter of the present invention is limited to the following embodiments, and all technologies realized based on the above contents of the present invention belong to the scope of the present invention.
Embodiment 1
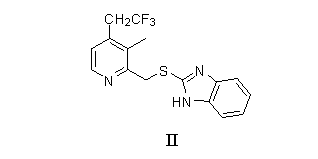
[0067] With 0.353 grams of 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfanyl]-1H-benzimidazole and 0.197 grams N,N'-dibenzyl-(R,R)-tartaric acid diamide was heated to 70°C in 2 ml of toluene, and stirred for 15 minutes. Then it was lowered to 60°C, and 0.09 ml of tetraisopropoxytitanium was added under heat preservation, and after heat preservation and stirring for 1 hour, 1.8 mg of water was added, and heat preservation and stirring for 1 hour. The temperature was lowered to 30°C, and 0.18 ml of phenylisopropyl hydroperoxide was added dropwise. After reacting at 30°C for 2 hours, the enantiomeric excess value of (R)-lansoprazole determined by HPLC was 91.7%, and the content was 73.8%.
Embodiment 2
[0069] With 0.353 grams of 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfanyl]-1H-benzimidazole and 0.197 grams N,N'-dibenzyl-(R,R)-tartaric acid diamide was heated to 70°C in 2 ml of 1,4-dioxane, and stirred for 15 minutes. Then it was lowered to 60°C, and 0.09 ml of tetraisopropoxytitanium was added under heat preservation, and after heat preservation and stirring for 1 hour, 1.8 mg of water was added, and heat preservation and stirring for 1 hour. The temperature was lowered to 30°C, and 0.18 ml of phenylisopropyl hydroperoxide was added dropwise. After reacting at 30°C for 2 hours, the enantiomeric excess value of (R)-lansoprazole determined by HPLC was 43.7%, and the content was 23.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com