Aptamer of docetaxel, aptamer derivative and use thereof
A nucleic acid aptamer and docetaxel technology, which can be applied to preparations for in vivo tests, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problem of no nucleic acid aptamers being found. , to avoid adverse drug reactions, improve stability, and achieve the effect of suitable drug release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
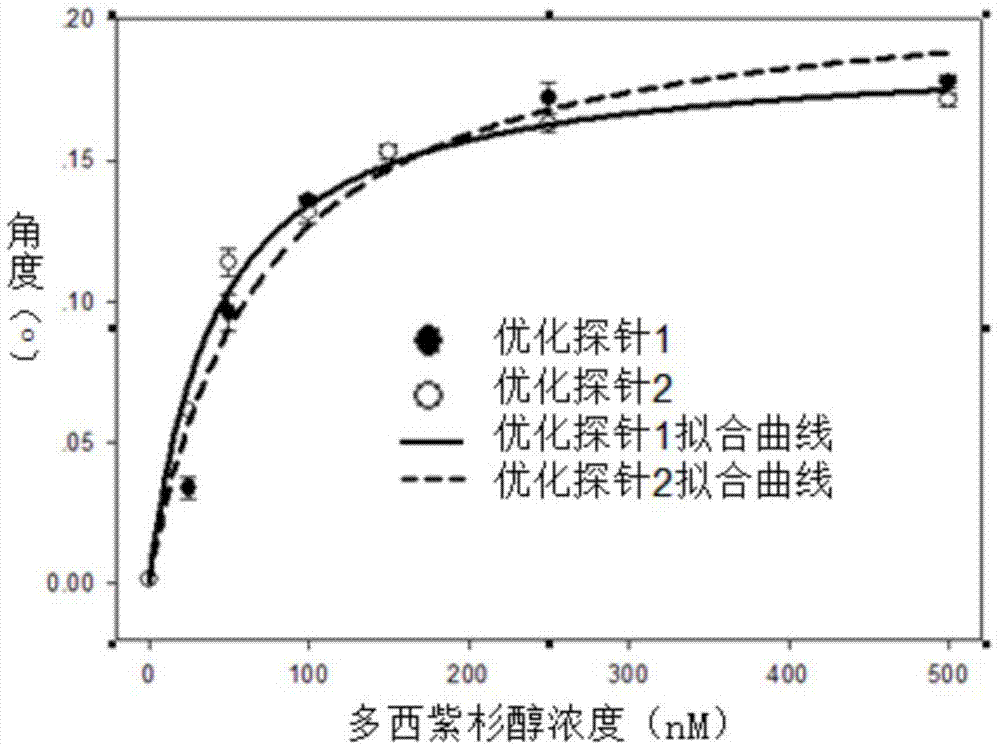
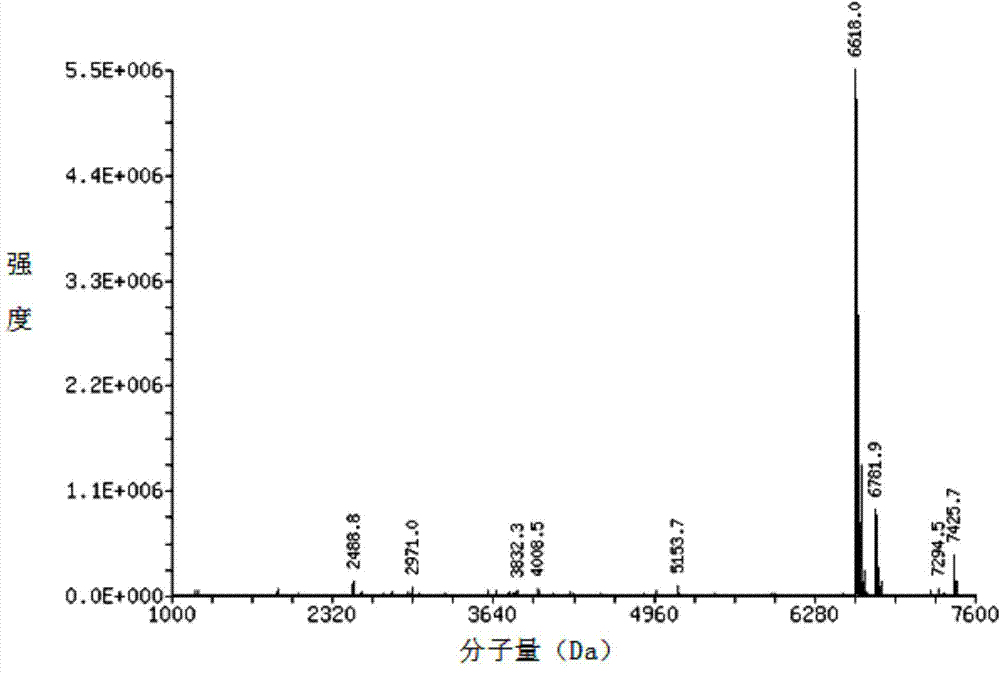
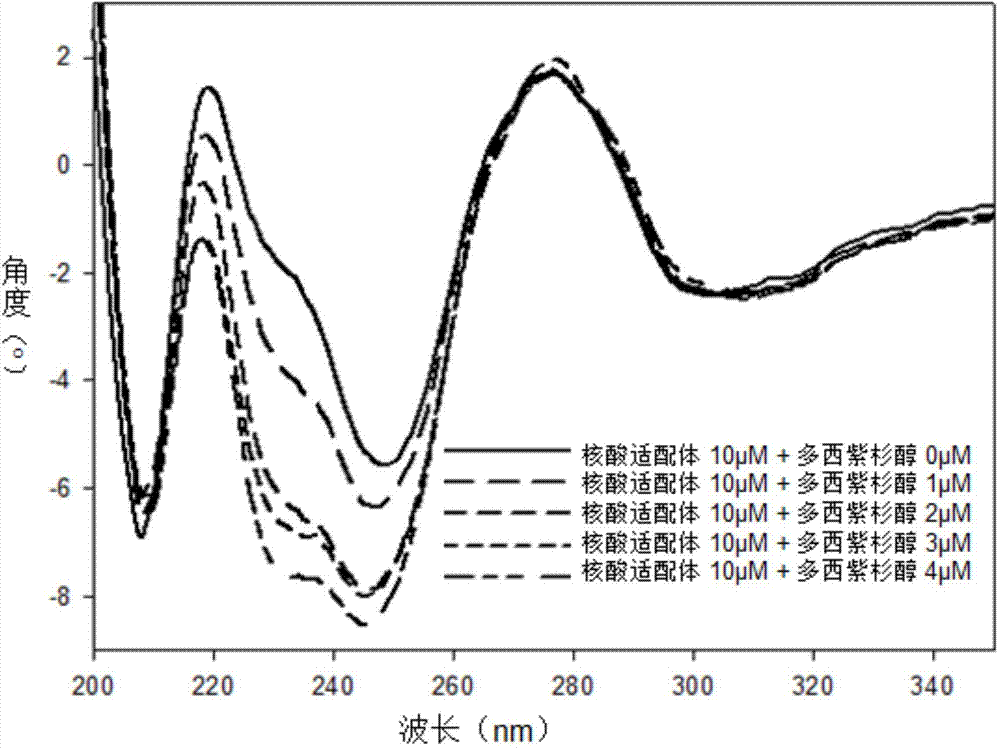
Image
Examples
Embodiment
[0035] A nucleic acid aptamer for docetaxel of the present invention, the nucleotide sequence of the nucleic acid aptamer includes a DNA fragment shown in the following sequence, that is, a characteristic sequence capable of specifically binding docetaxel:
[0036] 5’-TTCCAAACGTCCACCGCCAAAA-3’
[0037] (It can also be 5’-AAAACCGCCACCTGCAAACCTT-3’).
[0038] The nucleotide sequence of the aforementioned nucleic acid aptamer is selected from naturally occurring or artificially synthesized sequences, or the same sequence from any other source.
[0039] A nucleic acid aptamer sequence of docetaxel, the sequence of the nucleic acid aptamer contains the same sequence as all the nucleotides of the above-mentioned characteristic sequence.
[0040] A certain position on the nucleotide sequence of the above-mentioned nucleic acid aptamer can be phosphorylated, oxymethylated, methylated, amination, thiolated or isotopelated.
[0041] The nucleotide sequence of the aforementioned nucleic acid aptame...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com