A kind of synthetic method of azetidinone
A technology of azetidinone and synthesis method, applied in the direction of organic chemistry, can solve the problems of unstable reaction, affecting yield, easy ring-opening decomposition, etc., and achieve the effect of reducing cost, reducing emission and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
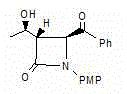
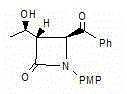
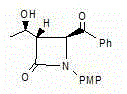
[0036] An azetidinone, the chemical name is: (3S,4S)-3-[(1R)-hydroxyethyl]-4-benzoyl-1-p-methoxyphenyl-2-azacyclic ring Butanone (compound Ⅰ), the structure is as follows,
[0037]
[0038] Compound I.
[0039] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butanamide (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0040] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 25°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 45°C, and Insulate and stir the reaction for 3-5 h, mon...
Embodiment 2
[0048]A kind of azetidinone, structure is the same as embodiment 1.
[0049] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butanamide (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0050] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 27°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 55°C, and Insulate and stir the reaction for 3-5 h, monitor the reaction to the end point by TLC, and wash the organic phase with 85 ml 2 mol / L hydrochloric acid, 230 ml 10% NaHCO 3 The aqueous...
Embodiment 3
[0058] A kind of azetidinone, structure is the same as embodiment 1.
[0059] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butanamide (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0060] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 23°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 45°C, and Insulate and stir the reaction for 3-5 h, monitor the reaction to the end point by TLC, and wash the organic phase with 85 ml 2 mol / L hydrochloric acid, 230 ml 10% NaHCO 3 The aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com