Anode active substance for lithium ion battery and preparation method thereof
A positive electrode active material and lithium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of high cost, insufficient stability, high synthesis temperature, etc., improve specific capacity and cycle performance, and improve electrochemical performance. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
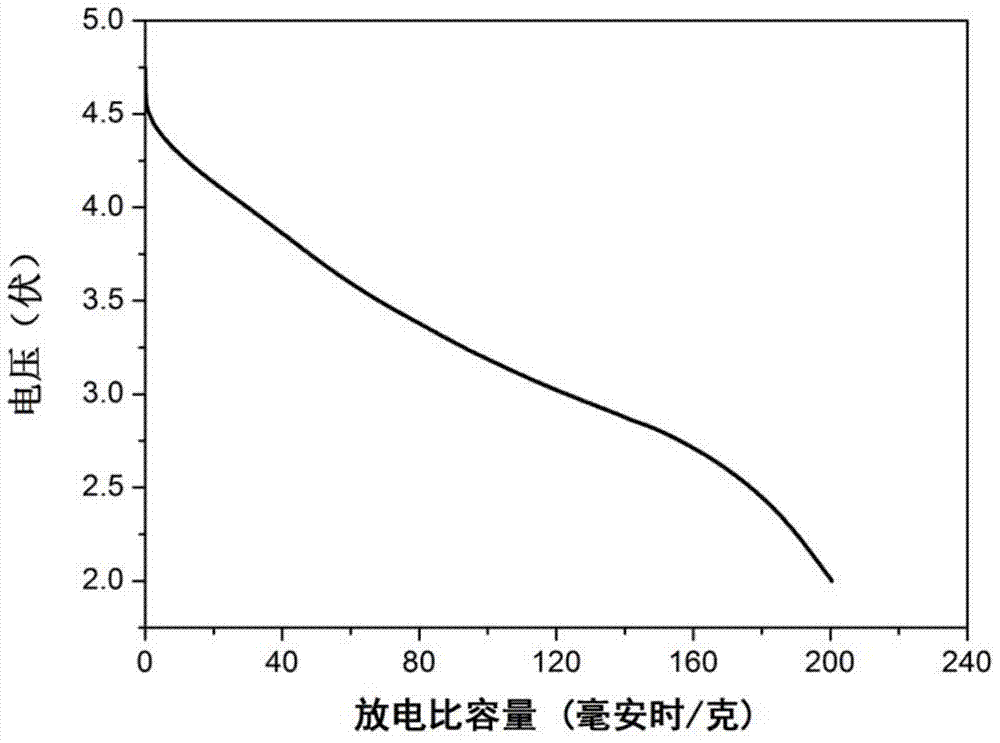
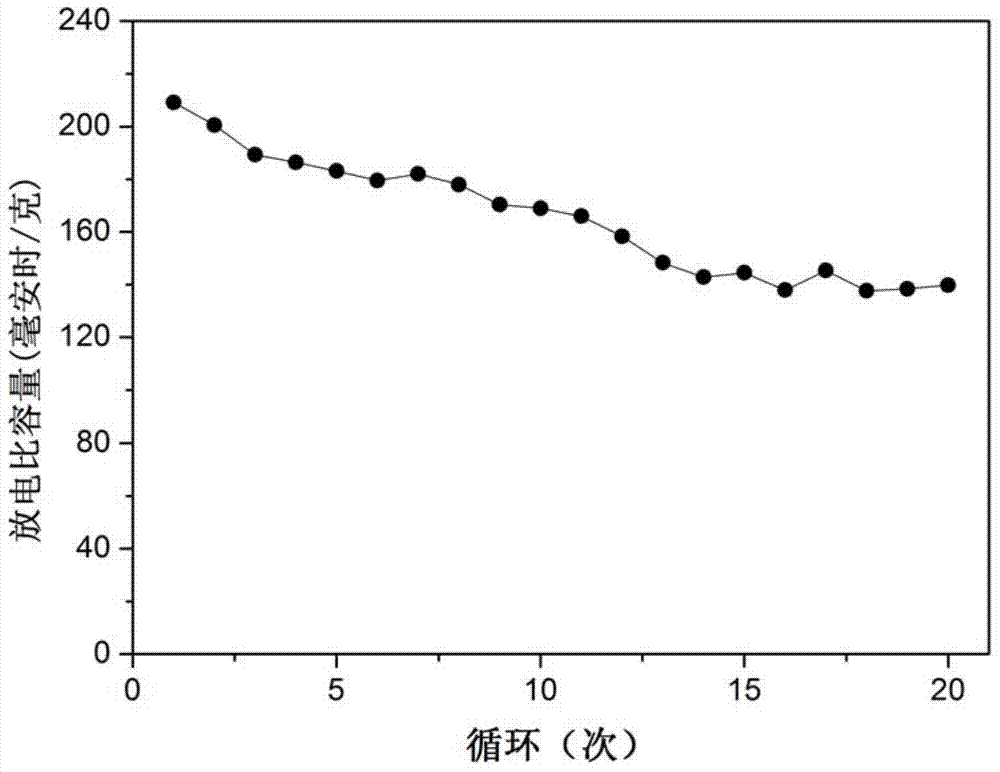
Examples
Embodiment 1
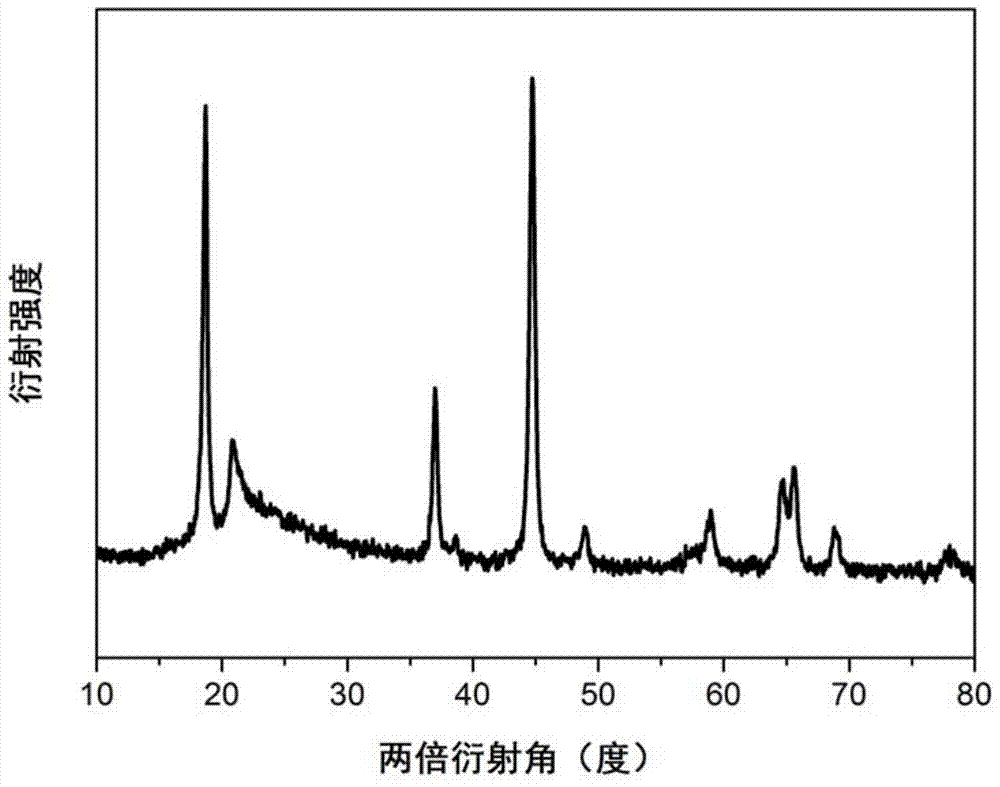
[0027] Weigh 2.9113gLi 2 CO 3 , 7.1576gMn (NO 3 ) 2Dissolve 0.0314g LiF in 10ml of deionized water to form a salt solution. Dissolve 12.6718g of citric acid in 10ml of deionized solution and add it to the aforementioned salt solution to form a mixed solution. Adjust the pH of the mixed solution to 8 with ammonia water, and then place the mixed solution on a magnetic heating Stirring was continued at a constant temperature of 60° C. on the stirrer for 8 hours to obtain a liquid precursor. Place the liquid precursor in a drying oven at 80°C for 16 hours, then dry at 150°C for 12 hours to obtain a solid precursor, place the solid precursor in a muffle furnace and calcinate at 500°C for 3 minutes, then grind it into a powder Precursor, heat the powdery precursor at 300°C for one hour and then heat it to 600°C at a heating rate of 2°C / min for 12h to obtain fluorine-doped Li 2 MnO 3 (Li 2 MnO 2.97 f 0.03 ).
[0028] The same method as Comparative Example 1 was used to prepa...
Embodiment 2
[0030] Weigh 5.3505gLiNO 3 , 4.5980gMnCO 3 And 0.0629gLiF was dissolved in 8ml deionized water to form a salt solution, 25.3435g citric acid was dissolved in 30ml deionized water and added to the aforementioned salt solution to form a mixed solution, the pH of the mixed solution was adjusted to 7 with ammonia water, and then the mixed solution was placed on a magnetic Stir continuously for 6 hours at a constant temperature of 80° C. on a heating stirrer to obtain a liquid precursor. Place the liquid precursor in a drying oven at 80°C for 24 hours, then dry at 130°C for 8 hours to obtain a solid precursor, place the solid precursor in a muffle furnace and calcinate at 500°C for 5 minutes, then grind it into a powder Precursor, heat the powdery precursor at 300°C for one hour and then heat it to 700°C at a heating rate of 4°C / min for 9h to obtain fluorine-doped Li 2 MnO 3 (Li 2 MnO 2.94 f 0.06 ).
[0031] The same method as Comparative Example 1 was used to prepare CR2025...
Embodiment 3
[0033] Weigh 5.2778gLiNO 3 , 6.9211gMn(CH 3 COO) 2 and 0.0943g LiF dissolved in 20ml of deionized water to form a salt solution, 38.0153g of citric acid was dissolved in 30ml of deionized water and added to the aforementioned salt solution to form a mixed solution, the pH of the mixed solution was adjusted to 9 with ammonia water, and then the mixed solution was placed on a magnetic Stir continuously for 6 hours at a constant temperature of 80° C. on a heating stirrer to obtain a liquid precursor. Place the liquid precursor in a drying oven at 80°C for 16 hours, then dry at 150°C for 8 hours to obtain a solid precursor, place the solid precursor in a muffle furnace and calcinate at 450°C for 7 minutes, then grind it into a powder Precursor, heat the powdery precursor at 300°C for 2 hours and then heat it to 600°C at a heating rate of 3°C / min for 15h to obtain fluorine-doped Li 2 MnO 3 (Li 2 MnO 2.91 f 0.09 ).
[0034] The same method as Comparative Example 1 was used t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com