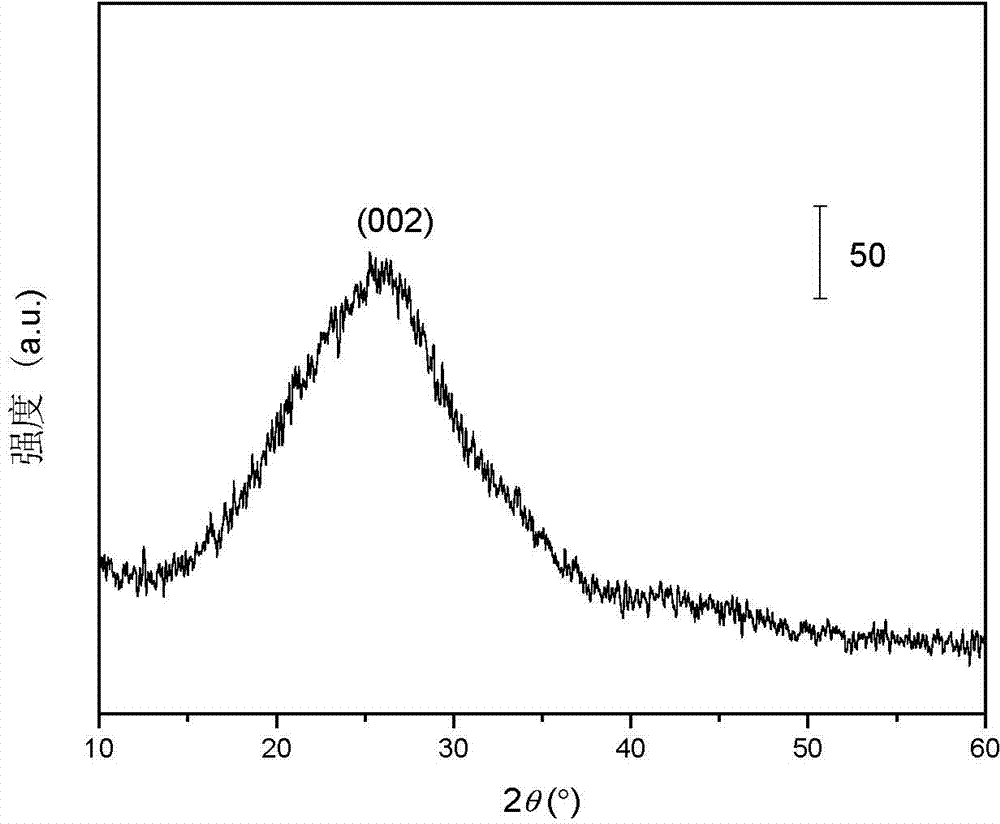
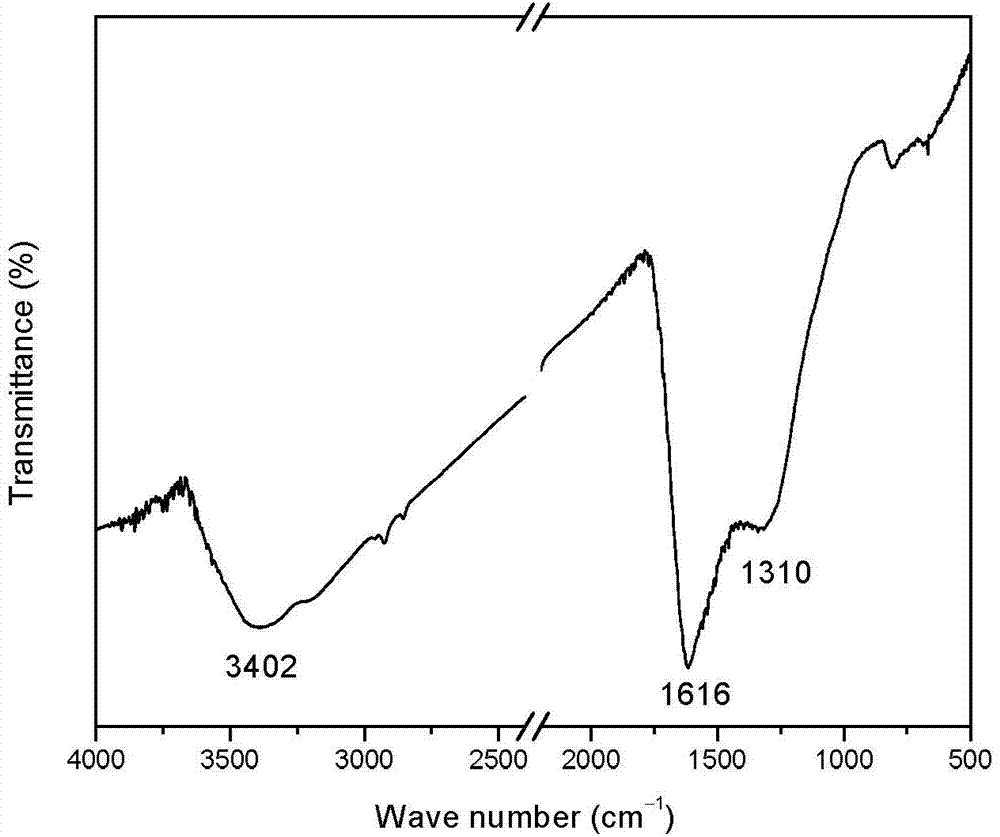
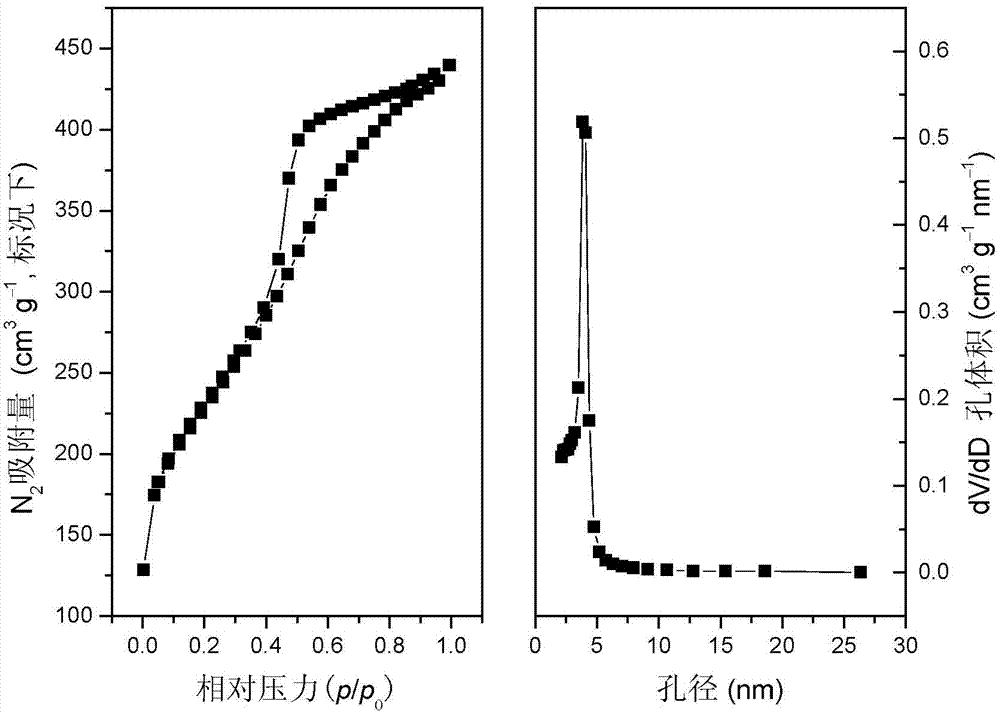
Preparation method of mesoporous graphite phase carbon nitride material
A graphite phase carbon nitride and mesoporous technology, applied in the direction of nitrogen and non-metallic compounds, can solve the problems of high price, small specific surface area of products, and high toxicity of raw materials, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) the urotropine of 5 mass parts is dissolved in the water of 10 mass parts, obtains the urotropine aqueous solution;
[0031] (2) Immerse 0.5 parts by mass of mesoporous SBA-15 in the urotropine aqueous solution of (1), and stir for 3 hours to form a white colloidal substance;
[0032] (3) Remove excess urotropine aqueous solution by centrifugation, and then dry the white solid in an oven at 50°C for 2 hours;
[0033] (4) Grind the obtained white powder, transfer it to a crucible with a cover, and roast it in a muffle furnace. -1 The heating rate increased to 550°C, and then kept at this temperature for 2 hours, and black powder was obtained after cooling down;
[0034] (5) Disperse the black powder in excess HF (~4mol L -1 ), stirred for 1–2 days to remove the silica template
[0035] (6) Wash the sample obtained in (5) with 50 parts by mass of water and ethanol, and finally dry it in an oven at 60°C for 2 hours to obtain the mesoporous g-C 3 N 4 .
[0036] Th...
Embodiment 2
[0038] (1) the urotropine of 6 mass parts is dissolved in the water of 10 mass parts, obtains the urotropine aqueous solution;
[0039] (2) Immerse 0.6 parts by mass of mesoporous SBA-15 in the urotropine aqueous solution of (1), and stir for 3.5 hours to form a white colloidal substance;
[0040] (3) Remove excess urotropine aqueous solution by centrifugation, and then dry the white solid in an oven at 50°C for 2.5 hours;
[0041] (4) Grind the obtained white powder, transfer it to a crucible with a cover, and roast it in a muffle furnace. -1 The heating rate increased to 550°C, and then kept at this temperature for 2 hours, and black powder was obtained after cooling down;
[0042] (5) Disperse the black powder in excess HF (~4mol L -1 ), stirred for 1–2 days to remove the silica template
[0043] (6) Wash the sample obtained in (5) with 70 parts by mass of water and ethanol, and finally dry it in an oven at 60°C for 2 hours to obtain the mesoporous g-C 3 N 4 .
[0044...
Embodiment 3
[0046] (1) the urotropine of 8 mass parts is dissolved in the water of 10 mass parts, obtains the urotropine aqueous solution;
[0047] (2) Immersing 1.0 parts by mass of mesoporous MCF material in the urotropine aqueous solution of (1), and stirring for 4 hours to form a white colloidal substance;
[0048] (3) Remove excess urotropine aqueous solution by centrifugation, and then dry the white solid in an oven at 50°C for 3 hours;
[0049] (4) Grind the obtained white powder, transfer it to a crucible with a cover, and roast it in a muffle furnace. -1 The heating rate increased to 550°C, and then kept at this temperature for 2 hours, and black powder was obtained after cooling down;
[0050] (5) Disperse the black powder in excess NH 4 HF 2 (~4mol L -1 ), stirred for 1–2 days to remove the silica template
[0051] (6) Wash the sample obtained in (5) with 100 parts by mass of water and ethanol, and finally dry it in an oven at 60°C for 2 hours to obtain the mesoporous g-C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com