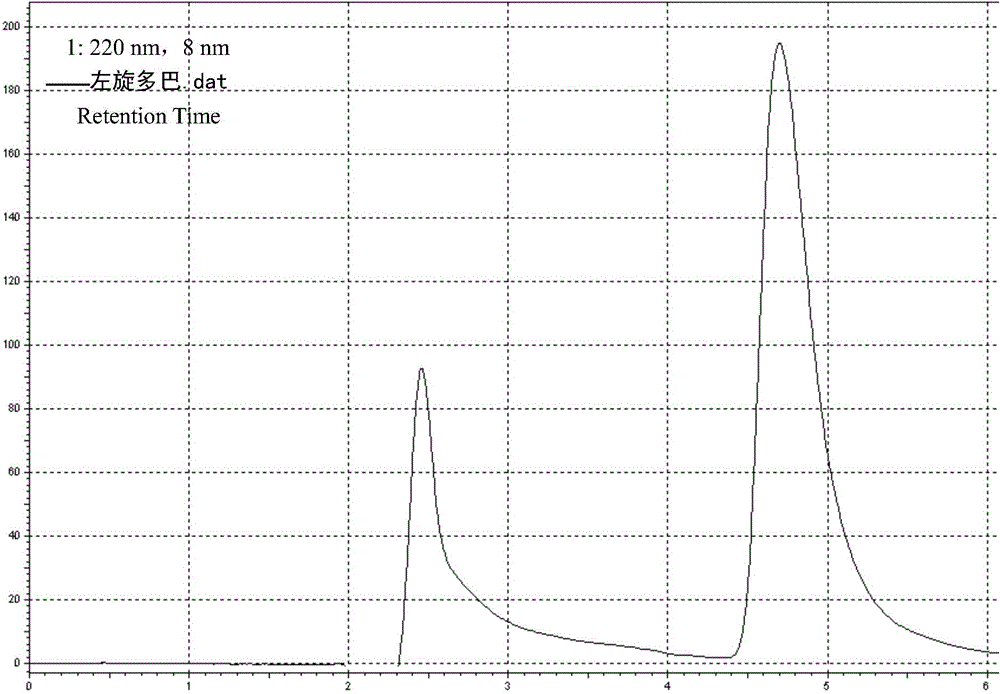
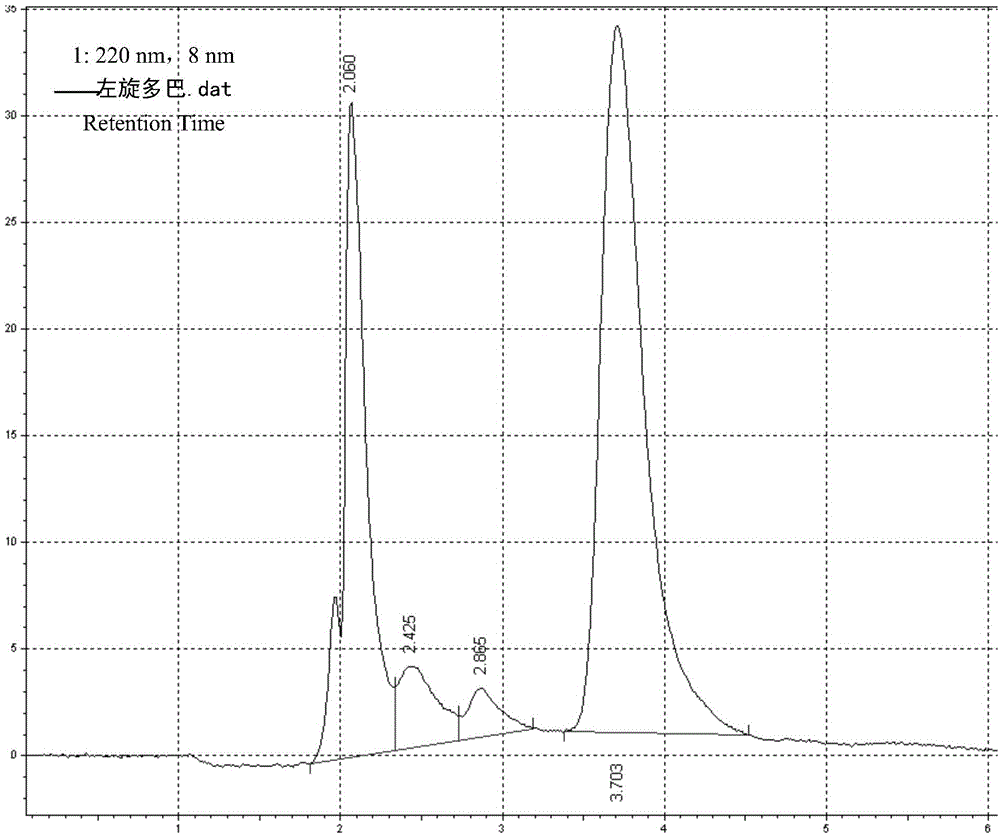
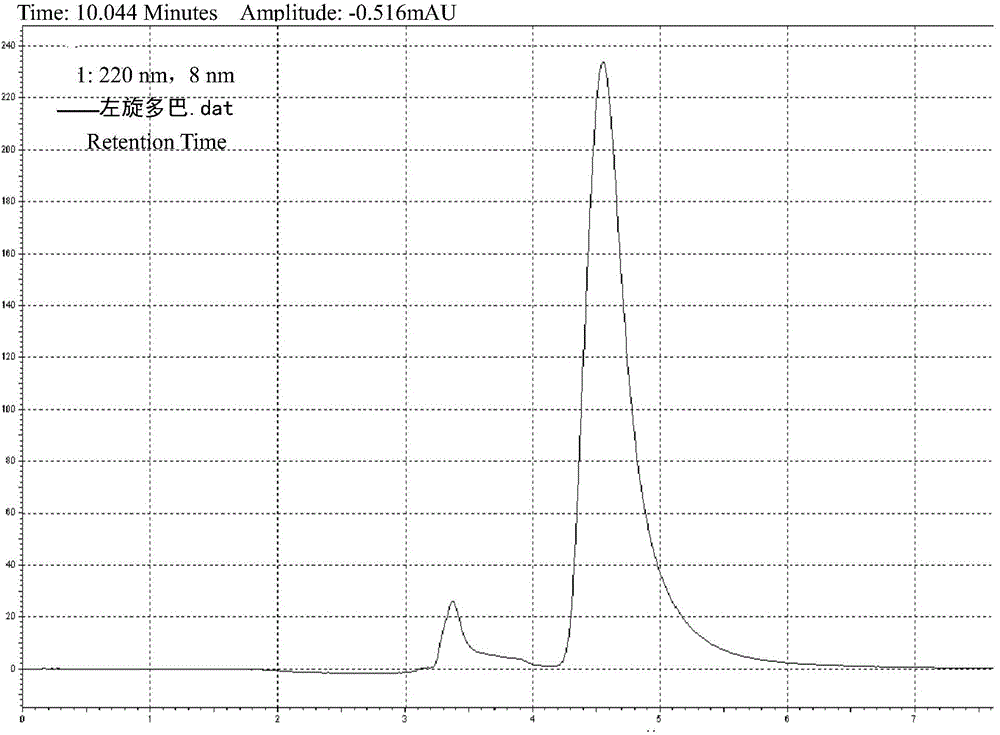
Levodopa / benserazide hydrochloride compound sustained-release suspension and preparation method thereof
A technology of benserazide hydrochloride and levodopa, which is applied in directions such as pharmaceutical formulations, medical preparations containing active ingredients, and emulsion delivery, to achieve the effects of solving difficulty in swallowing and being convenient to take.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 2. Impregnation of drug resin
[0034] Take an appropriate amount of drug-loaded resin, add it to 20% PEG6000 aqueous solution, stir for 0.5 hour, dry and sieve to obtain impregnated drug resin.
[0035] 3. Preparation of Drug Resin Microcapsules
[0036] The acrylic resin is dissolved in acetone, then the impregnated drug-loaded resin and PEG400 are added, stirred for a certain period of time, and the latter two are evenly dispersed to form a suspension. Add this suspension to the homogeneously mixed liquid paraffin and span80 mixture under stirring conditions, then stir in a water bath at 30°C for 4 hours, filter the obtained microcapsules with suction, wash with petroleum ether, and store at 40°C Drying under heat to obtain drug resin microcapsules.
[0037] 4. Preparation of Drug Resin Microcapsule Suspension
[0038] Take a certain amount of drug-resin microcapsules, an appropriate amount of suspending agent (one or more of PVP, HPMC, tragacanth gum, Carbopol, A...
Embodiment 1
[0046] 100 mg of the drug-loaded resins were taken, respectively added to 10 ml of 20% PEG6000 aqueous solution, stirred for 0.5 hour, dried and sieved to obtain impregnated drug resins.
[0047] 3. Preparation of Drug Resin Microcapsules
[0048] Dissolve 40mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated levodopa resin and 1ml of PEG400, and magnetically stir for a certain period of time to disperse the latter two evenly to form a suspension. Add this suspension to the mixed solution of 24ml liquid paraffin and 1ml span80 which has been mixed homogeneously under the condition of stirring, then stir in a water bath at 30°C for 4 hours, filter the obtained microcapsules with suction, wash with petroleum ether, and in Dry at 40°C to obtain drug resin microcapsules.
[0049] Dissolve 50mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated benserazide hydrochloride resin and 1ml of PEG400, and stir magnetically for a certain period of time to d...
Embodiment 2
[0055] 2. Impregnation of drug resin
[0056] Take 100 mg of the drug-loaded resin, add 10 ml of 20% PEG6000 solution, stir for 0.5 hour, dry and sieve to obtain the impregnated drug resin.
[0057] 3. Preparation of Drug Resin Microcapsules
[0058] Dissolve 60mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated levodopa resin and 1ml of PEG400, and stir for a certain period of time to disperse the latter two evenly to form a suspension. Add this suspension to the mixed solution of 24ml liquid paraffin and 1ml span80 which has been mixed homogeneously under the condition of stirring, then stir in a water bath at 30°C for 4 hours, filter the obtained microcapsules with suction, wash with petroleum ether, and in Dry at 40°C to obtain drug resin microcapsules.
[0059] Dissolve 75mg of acrylic resin in 8ml of acetone, then add 200mg of impregnated benserazide hydrochloride resin and 1ml of PEG400, and stir for a certain period of time to disperse the latter two e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com