Near-infrared wide-spectrum metal complex dye and preparation method thereof
A metal complex and wide-spectrum technology, applied in the field of dye-sensitized solar cells, can solve the problems of less dyes and low absorption capacity, and achieve the effect of increasing the spectral response range and improving the photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
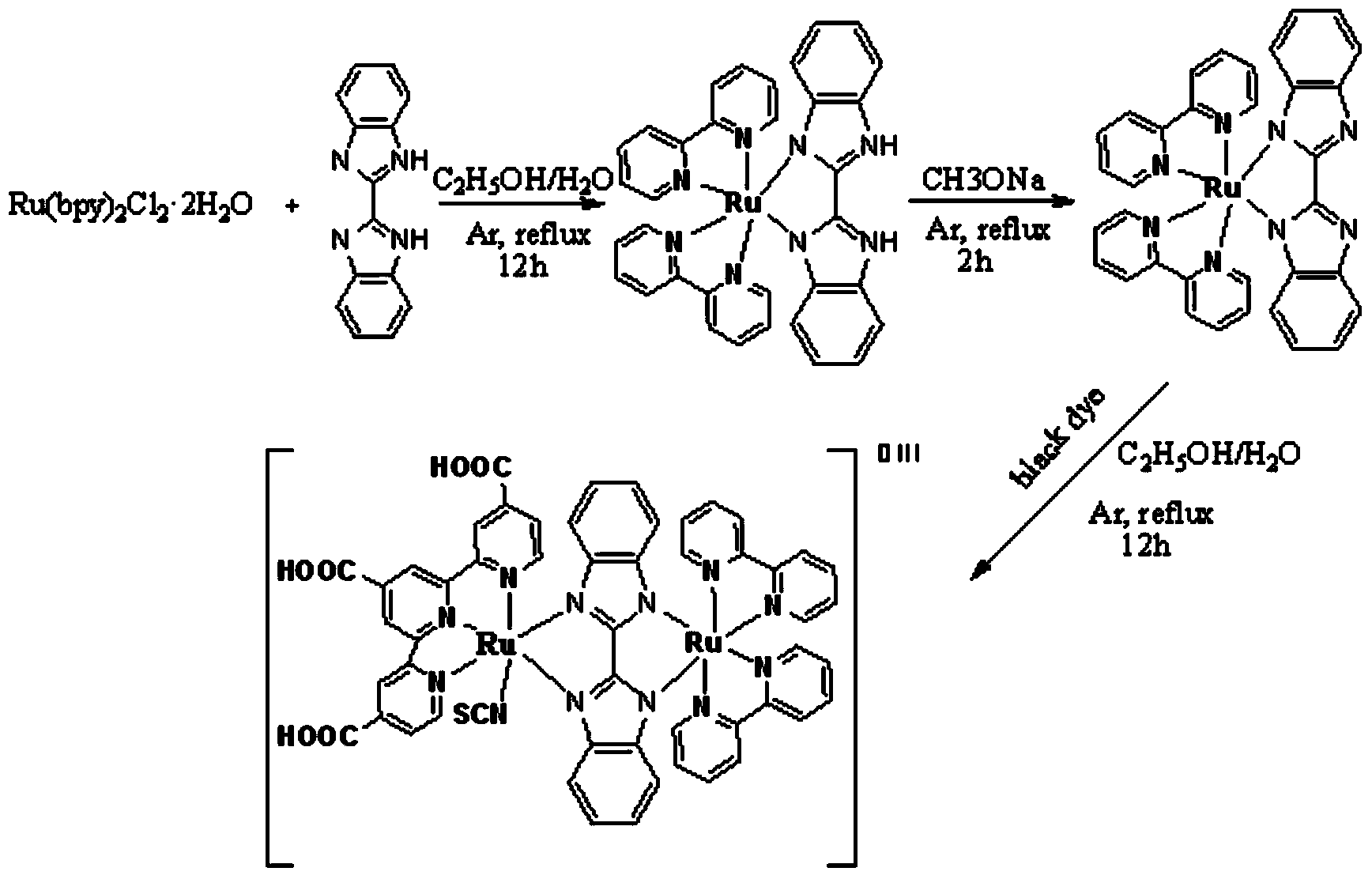
[0041] dye-1: Ru(bpy) 2 (Bibz)(tcpy)(SCN)Ru
[0042] The structure of dye-1 is The metal ion is Ru, and the ligand structure around the metal ion is and Two types, where the ligand structure There are no R, R' substituents, R 1 , R 2 , R 3 is a carboxyl substituent; the bridging ligand is Type, the bridging ligand does not have R, R' substituents.
[0043] Although the ligand structure in this example There are no R, R' substituents, however, in similar structures, the R, R' substituents of the dye can be carboxyl (-COOH), and other structures, where R 1 , R 2 , R 3 , R 4 , R 4’ Can be -X, Me-, MeO-, -COOH, -SO 3 H. At least one of them, X can be any one of F, Cl, Br, I; or the R, R' substituent of the dye has no R 1 , R 2 , R 3 , R 4 , R 4’ substituent group.
[0044] metal ligand structure The substituent R 1 , R 2 , R 3 Can also be -X, Me-, MeO-, -COOH, -SO 3 H. At least one of, X is any one of F, Cl, Br, I; R 1 , R 2 , R 3 It ...
Embodiment 2
[0051] dye-2: [Os(bpy) 2 (Bi)(dcbpy) 2 Os]Cl 4
[0052] The structure of dye-2 is The metal ion is Os, and the ligands around the metal ion are type, and some ligands contain carboxyl substituents, and some have no substituents; the bridging ligands are type, and the bridging ligand has no substituents. Although the bridging ligand has no substituents in this example, in a similar structure, R and R' of this type of bridging ligand can be Any one of them, and the bridging ligand can have one, two, three or four R or R' substituents, wherein R 1 , R 2 , R 3 , R 4 , R 4’ The substituents are the same as in Example 1.
[0053] Dye [Os(bpy) 2 (Bi)(dcbpy) 2 Os]Cl 4 The synthetic route of Figure 4 Shown, its preparation method is as follows:
[0054] ⑴.[Os(bpy) 2 (BiH 2 )] 2+ The synthesis of: under Ar gas conditions, add Os(bpy) in the flask 2 Cl 2 2H 2 O (0.19mmol) and BiH 2 (0.19mmol), then add 30ml of ethanol and water mixture (volume ratio = 1:1), an...
Embodiment 3
[0058] Dye-3: Ru(tcpy)(dpdpz)(bpy) 2 Ru
[0059] The structure of dye-3 is Its metal ion ligand is the same as dye-1, and its bridging ligand is type, and the bridging ligand has no substituents.
[0060] Bridging ligands with similar structures can also be
[0061] Dye Ru(tcpy)(dpdpz)(bpy) 2 The synthetic route of Ru is as follows Figure 5 Shown:
[0062] ⑴. Synthesis of Ru(tm-tcpy)(dpdpz)Cl: Add Ru(tm-tcpy)Cl to the flask under Ar gas condition 3(0.19mmol) and dpdpz (0.19mmol), then add 30ml of a mixture of ethanol and water (volume ratio = 3:1), add 1ml of triethylamine under stirring, and reflux for more than 12 hours. After the reaction, the solution is brown-black , spin-dried, and can be directly used in the next step without purification.
[0063] ⑵Ru(tm-tcpy)(dpdpz)(bpy) 2 Synthesis of Ru: Dissolve an appropriate amount of Ru(bpy)2Cl2 in 30ml of acetone, add an appropriate amount of AgClO4 under Ar gas, then reflux for 2 hours, filter after cooling, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com