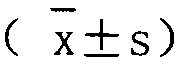Real-data-based simulative RCT (randomized controlled trial) analysis method
An analysis method and real data technology, applied in the fields of electrical digital data processing, special data processing applications, instruments, etc., can solve the problems of difficult differences in therapeutic effects, limited extrapolation, and small number of studies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
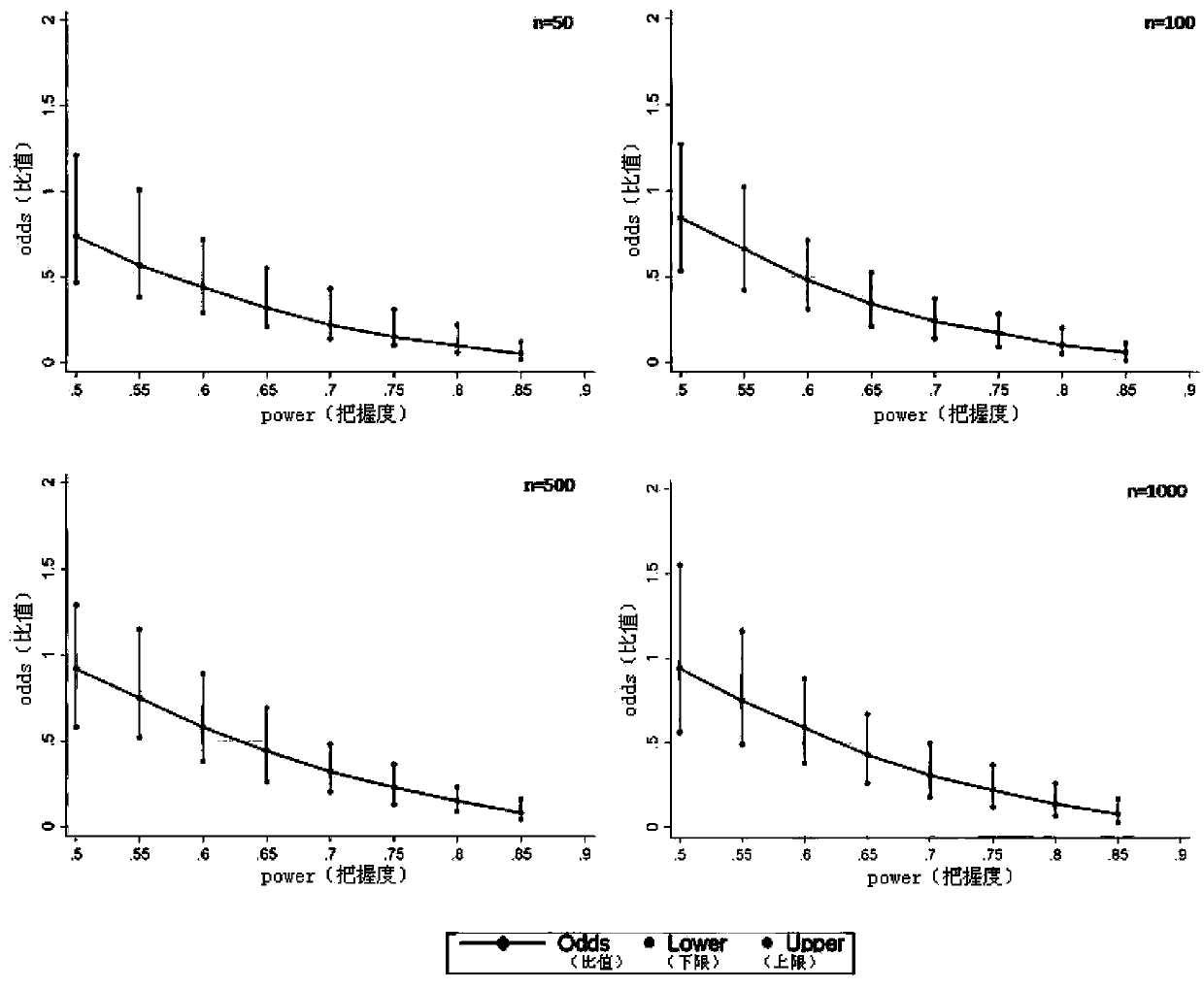
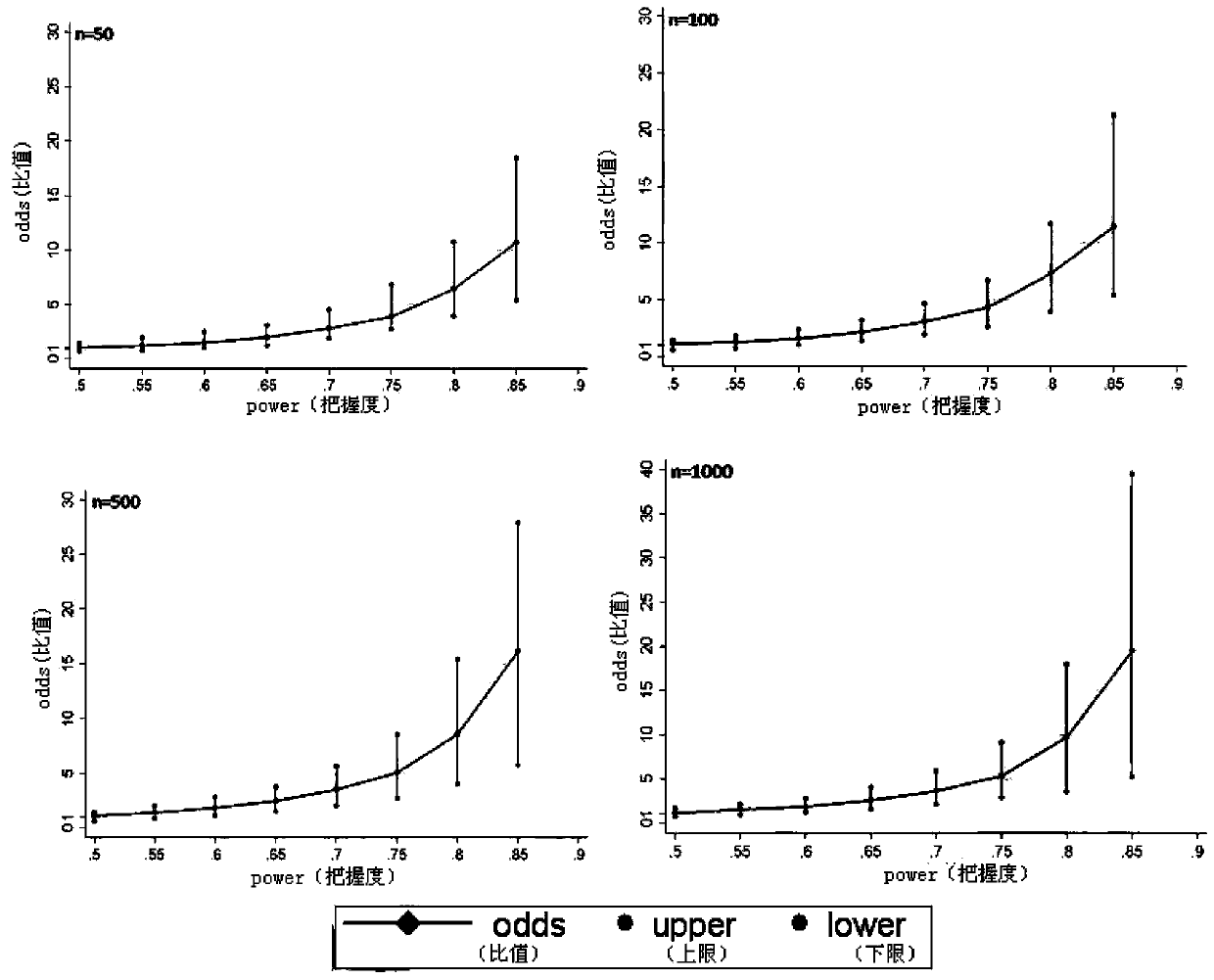
[0021] The programming tool of Statall.0 statistical analysis software is used for specific implementation, and the complete program is formed by using Statall.0 software programming, and the corresponding analysis is carried out by using simulated data. The actual application does not include the process of creating a simulated database. This analysis method can directly perform repeated randomized grouping, Odds and 95% CI calculation, and inter-group balance analysis of confounding factors for different real clinical data, and finally provide Odds and 95% CI, as well as inter-group balance of confounding factors The results of the comparison (probability of imbalance between groups of confounding factors) are used as the basis for judging the effects of different interventions; the specific steps are as follows:
[0022] 1. Create a database
[0023] Using PS (power and sample size program) software to calculate the size of E / S corresponding to Power under different sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com