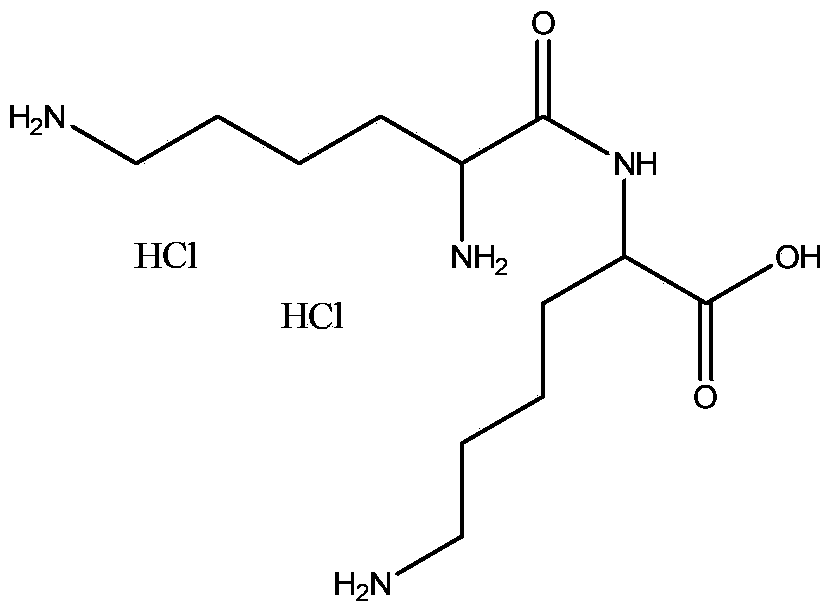
Stable formulations of a hyaluronan-degrading enzyme
A technology of hyaluronan and hyaluronidase is applied in the field of stable preparations of hyaluronan degrading enzymes, and can solve the problems of hypoglycemia, prolonged action time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0701] Preparation of Insulin and Insulin Analog Stocks
[0702] A. Regular insulin
[0703] For regular insulin, weigh the powder (Organon Insulin API, Recombinant Human Insulin SIHR143) and mix it with an appropriate amount of water until the solution contains approximately 10-25 mg / mL of insulin. 1 M HCl was added to the turbid mixture to a final concentration of HCl of 20 mM. The solution was mixed gently with a stir bar until the insulin was completely dissolved, and 250 mM Tris, pH 10.7 (Trizma, cat# T6066, Sigma) was added to a final Tris concentration of 20 mM. Insulins were formulated as described in each of the individual examples below using 1 M NaOH to adjust the pH followed by the addition of water. This insulin contains approximately 13 μg / mL zinc.
[0704] B. Insulin analogues
[0705] For insulin analogs (insulin aspart or insulin lispro), combine 12 vials (10 mL each) of commercially available product (insulin lispro: Eli Lilly (Insulin lispro) 100U / mL, ...
Embodiment 2
[0707] Determination of hyaluronidase activity of rHuPH20
[0708] The hyaluronidase activity of rHuPH20 (obtained by expression and secretion of a nucleic acid encoding amino acids 36-482 of SEQ ID NO: 1 in CHO cells) was determined using a nephelometric assay. In the first 2 assays (A and B), the hyaluronidase activity of rHuPH20 was measured by incubating soluble rHuPH20 with sodium hyaluronate (hyaluronic acid), followed by precipitation of undigested by addition of acidified serum albumin of sodium hyaluronate. In the third assay (C), rHuPH20 hyaluronidase activity was measured based on the formation of an insoluble precipitate when hyaluronic acid (HA) was combined with cetylpyridinium chloride (CPC). In all assays containing 600 U / mL rHuPH20 (5 μg / mL), the acceptance criterion was an enzyme activity above 375 U / mL.
[0709] A. Microturbidimetry
[0710] In this assay, the hyaluronidase activity of rHuPH20 is measured by incubating soluble rHuPH20 with sodium hyaluron...
Embodiment 3
[0728] RP-HPLC
[0729] In this example, reverse phase-HPLC (RP-HPLC) was used to determine the apparent solubility of insulin and insulin analogs and the percent purity of rHuPH20. Apparent solubility was measured as percent insulin recovery relative to the initial formulation / condition.
[0730] reference standard
[0731] For regular insulin, use (regular insulin, 100U) as a standard. For insulin lispro, reconstitute 1 vial of USP insulin lispro with 1.72 mL of 0.01N HCl to obtain a 3.5 mg / mL USP insulin lispro (100 U / mL) reference standard. For insulin aspart, reconstitute 1 vial of EP insulin aspart with 1.00 mL of 0.01N HCl to obtain a 3.89 mg / mL EP insulin aspart reference standard. For rHuPH20, use 3 reference standards, each containing a 50 μg / mL rHuPH20 sample (Lot #HUB0701EB ) produced 2.5 μg / mL, 5.0 μg / mL or 7.5 μg / mL rHuPH20.
[0732] Preservative Standards
[0733] Three preservative reference standards were used, each containing 0.05% m-cresol / phenol, 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com