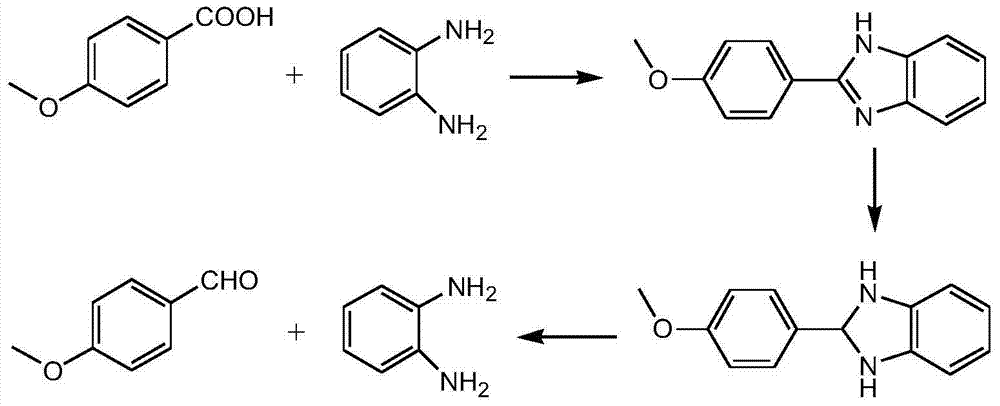
A kind of method that p-methoxybenzoic acid prepares p-methoxybenzaldehyde
A technology of p-methoxybenzaldehyde and methoxybenzoic acid, applied in the field of daily chemical industry, can solve problems such as expensive reagents, and achieve the effects of saving production costs, mild reaction conditions, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 152kg of p-methoxybenzoic acid, 300kg of o-xylene, and 118.8Kg of o-phenylenediamine into the reaction kettle, heat up to reflux (reaction temperature is 135-145° C.), and continuously separate the water produced by the reaction.
[0050] After 4.5 hours of reaction, the reaction was completed, and the temperature was directly lowered to 10-15° C., and filtered with suction, and the filtrate was used for the next batch. HPLC analysis filter cake content 99.5%, yield 98.5%.
[0051] HPLC-MS analysis molecular weight is 224, through 1 H-NMR detection confirmed that it was p-2-(4-methoxyphenyl)benzimidazole.
Embodiment 2
[0053] Add 152kg of p-methoxybenzoic acid, 300kg of m-xylene, and 118.8Kg of o-phenylenediamine into the reaction kettle, heat up to reflux (reaction temperature is 136-144° C.), and continuously separate the water produced by the reaction.
[0054] After 6.5 hours of reaction, the reaction was completed, and the temperature was directly lowered to 10-15°C, and suction filtered, and the filtrate was used for the next batch. HPLC analysis filter cake content 99.2%, yield 96.5%.
[0055] HPLC-MS analysis molecular weight is 224, through 1 H-NMR detection confirmed that it was p-2-(4-methoxyphenyl)benzimidazole.
Embodiment 3
[0057] Add 152kg of p-methoxybenzoic acid, 300kg of p-xylene, and 118.8Kg of o-phenylenediamine into the reaction kettle, heat up to reflux (reaction temperature is 137-150° C.), and continuously separate the water produced by the reaction.
[0058] After 4.0 hours of reaction, the reaction was completed, and the temperature was directly lowered to 10-15° C., filtered with suction, and the filtrate was used for the next batch. HPLC analysis filter cake content 99.4%, yield 93.7%.
[0059] HPLC-MS analysis molecular weight is 224, through 1 H-NMR detection confirmed that it was p-2-(4-methoxyphenyl)benzimidazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com