Metal aluminium complex and application thereof to organic luminescent device
A metal aluminum and complex technology, applied in the field of organic electroluminescence devices, can solve the problems of lack of long-life transmission materials and host materials, and achieve the effects of high electron mobility, good stability and increased power efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
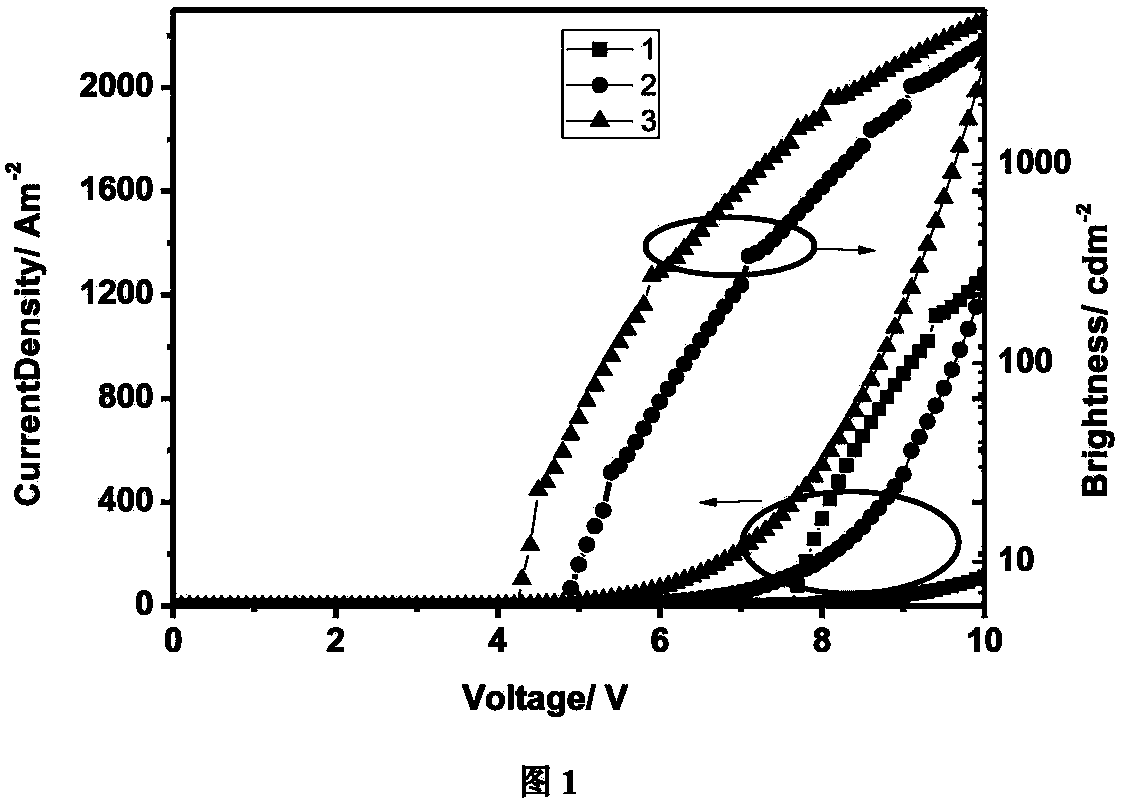
Image
Examples
Embodiment 1
[0055] Example 1: Compound Q1-1
[0056] Reaction formula:
[0057]
[0058] 4.1g (20mmol) of aluminum isopropoxide and 3.42g (20mmol) of 4-(3-pyridine)phenol were dissolved in 80ml of toluene, heated to reflux and stirred for 3 hours. Then 2-methyl-8-hydroxyquinoline was dissolved in 20 ml of toluene, and the solution was added dropwise into the reaction bottle, and the solution was heated and stirred under reflux to react overnight. Filter and wash with toluene to obtain a white flocculent solid. The final product was purified by partition sublimation to obtain 4.3 g of a yellowish solid with a yield of 42%.
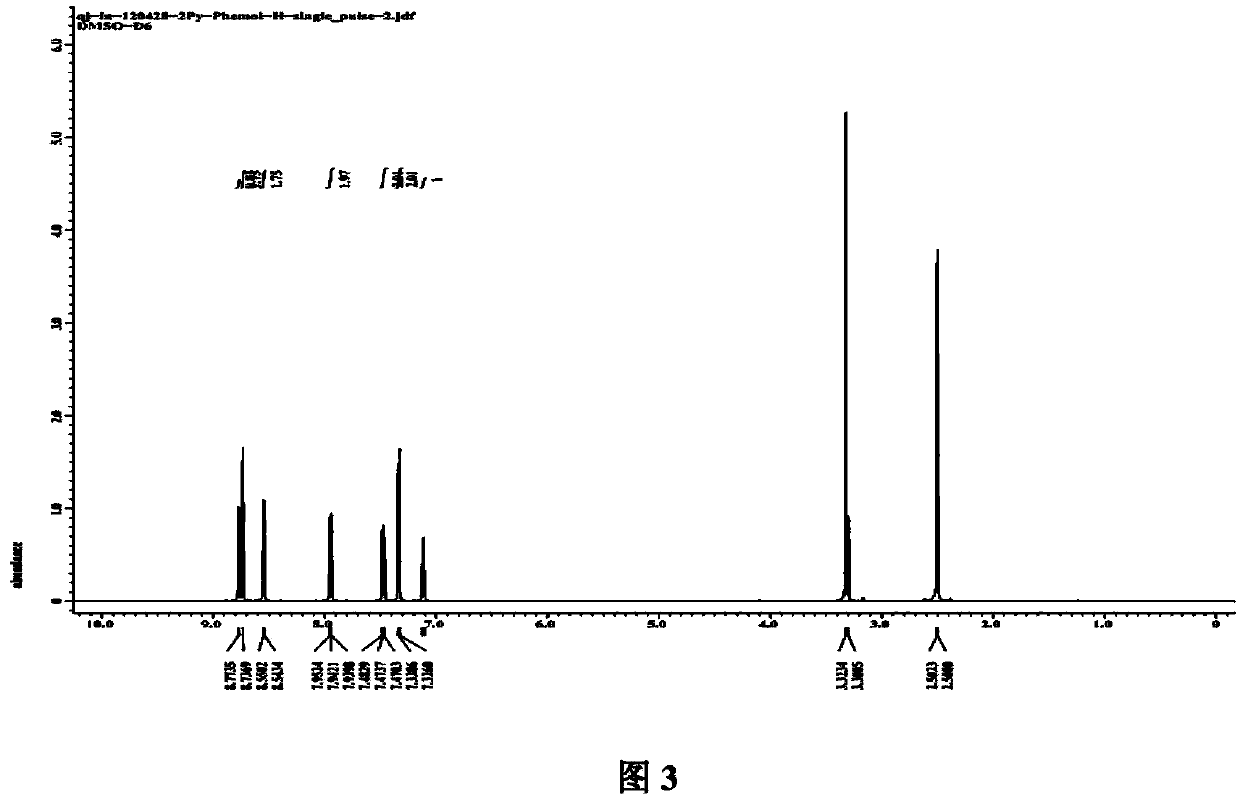
[0059] 1 H-NMR (CDCl 3 ,300MHz,δ[ppm]):2.73(s,6H),6.79~6.81(d,2H),6.89~6.92(m,2H),7.19~7.20(d,4H),7.37~7.39(m,4H ),7.60~7.62(d,2H),7.89~7.92(d,2H),8.65~8.67(d,2H).
[0060] ESI-MS[m / z]:514[M+H] + .Elemental analysis (C 31 h 24 AlN 3 o 3 ): Anal. Calcd.: C, 72.53; H, 4.71; N, 8.18. Found: C, 72.37; H, 4.36; N, 8.36.
Embodiment 2
[0061] Embodiment 2: Compound Q1-2
[0062] The synthesis of 3-(3-pyridine)phenol ligand can be obtained by referring to the literature (US20110039958A1). The synthesis of the complex refers to the synthesis method of Example 1, and the yield is 45%.
[0063] ESI-MS[m / z]:514[M+H] + .Elemental analysis (C 31 h 24 AlN 3 o 3 ): Anal. Calcd.: C, 72.53; H, 4.71; N, 8.18. Found: C, 72.44; H, 4.55; N, 8.00.
Embodiment 3
[0064] Embodiment three: compound Q1-3
[0065] The synthesis method is the same as in Example 1, except that 4-(3-pyridine)phenol is replaced by 2-(3-pyridine)phenol. The yield was 42%.
[0066] ESI-MS[m / z]:514[M+H] + .Elemental analysis (C 31 h 24 AlN 3 o 3 ): Anal. Calcd.: C, 72.53; H, 4.71; N, 8.18. Found: C, 72.37; H, 4.36; N, 8.36.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com