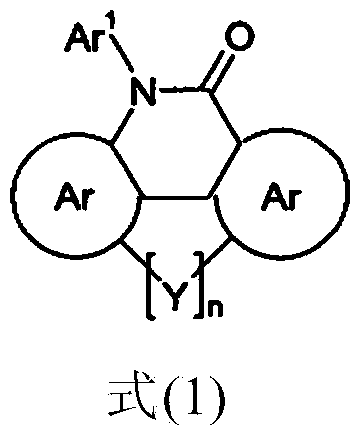
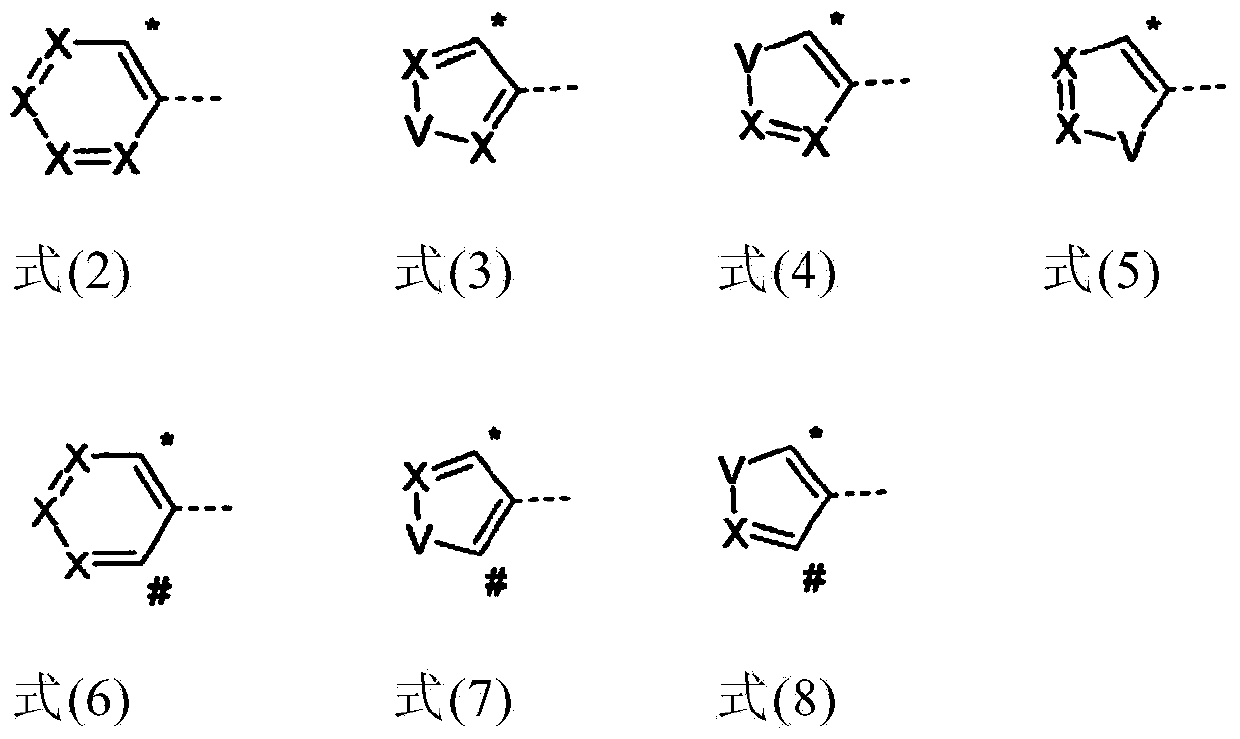
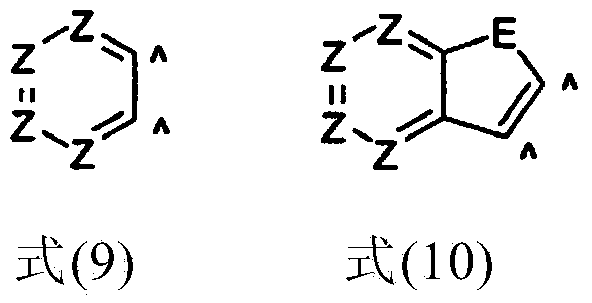
Organic electroluminescent device
A technology of electronic devices and groups, applied in electroluminescent light sources, electric solid-state devices, electric light sources, etc., can solve problems such as side reactions and electrochemical instability of light-emitting devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: 2-bromo-N,N-bis(biphenyl)benzylamine
[0142]
[0143] 9.7 g (0.243 mol) of 60% NaH in mineral oil were dissolved in 500 ml of dimethylformamide under protective atmosphere. 60 g (0.106 mol) of N,N-bis(4-biphenyl)amine was dissolved in 500 ml of DMF and added dropwise to the reaction mixture. After 1 hour at room temperature, a solution of 60.6 (242 mmol) 2-bromobenzyl bromide in 500 ml DMF was added dropwise. The reaction mixture was stirred at room temperature for 1 hour. Afterwards, the reaction mixture was poured onto ice and extracted three times with dichloromethane. The combined organic phases were washed with Na 2 SO 4 Dry and evaporate. The residue was extracted with hot toluene and recrystallized from toluene / n-heptane. Yield 80 g (93%).
[0144] The following compounds are similarly obtained:
[0145]
[0146]
Embodiment 2
[0147] Example 2: 5-biphenyl-4-yl-2-phenyl-5,6-dihydrophenanthridine
[0148]
[0149] Dissolve 47g (0.158mol) of 2-bromo-N,N-bisbiphenylbenzylamine in 500ml of dimethylformamide under a protective atmosphere. 17.3 g (0.075 mol) of benzyltrimethylammonium bromide and 31.28 g (0.226 mol) of potassium carbonate were added to the solution. Then add 5.08g (0.022mol) Pd(OAc) under protective gas 2 , and the mixture was stirred at 120 °C for 9 hours. Afterwards, the reaction mixture was cooled to room temperature and extracted with dichloromethane. The combined organic phases were washed with Na 2 SO 4 Dry and evaporate. The residue was recrystallized from n-heptane. Yield 51 g (84%).
[0150] The following compounds are similarly obtained:
[0151]
[0152]
Embodiment 3
[0153] Example 3: 5-biphenyl-4-yl-2-phenyl-6(5H)-phenanthridinone
[0154]
[0155] Dissolve 16g (0.039mol) of 5-biphenyl-4-yl-2-phenyl-5,6-dihydrophenanthridine in 300ml of dichloromethane, and add 62.13g (0.393mol) of potassium permanganate in portions to the solution, and the mixture was stirred at room temperature for two days. Afterwards, the residual potassium permanganate was filtered off, the solution was evaporated and purified by chromatography (eluent: heptane / dichloromethane, 5:1). The residue was recrystallized from n-heptane. Yield 14 g (88%).
[0156] The following compounds are similarly obtained:
[0157]
[0158]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com