Immunoglobulin fc variants
An immunoglobulin and globulin technology, applied in the field of immunoglobulin Fc variants, can solve the problems of inability to increase half-life and low biological activity, and achieve the effect of low administration frequency and increase in vivo half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Construction of vectors expressing immunoglobulin Fc fragments
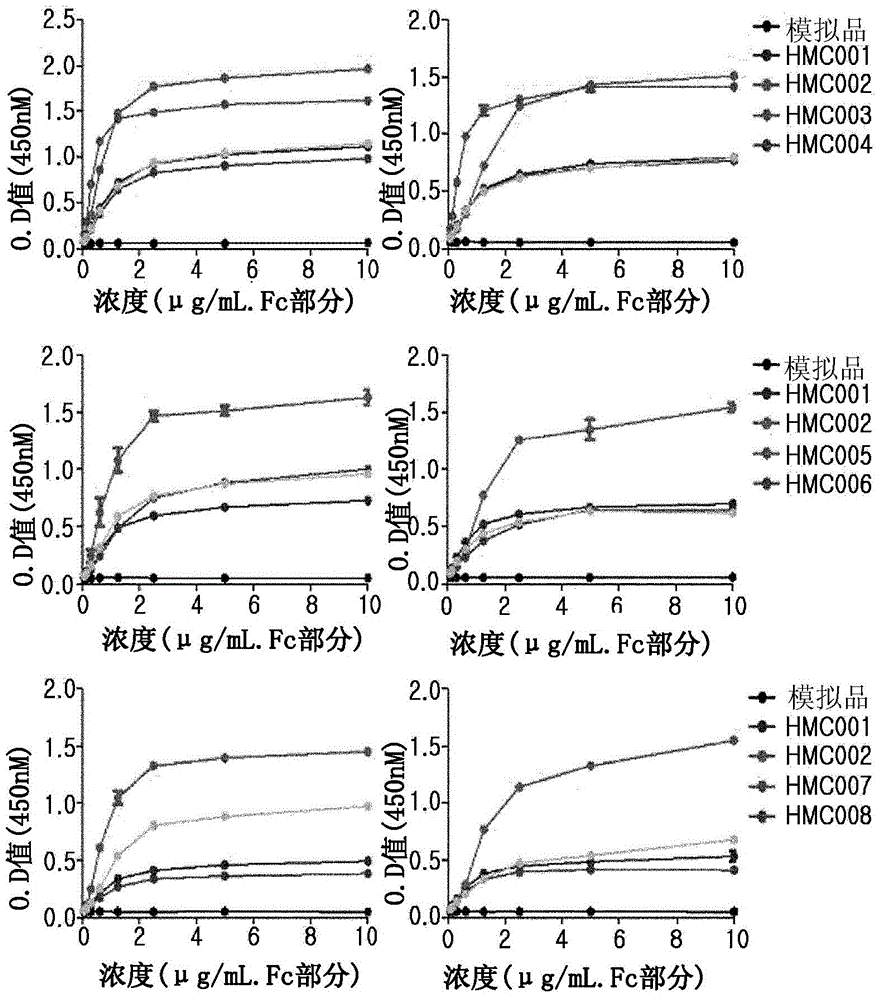
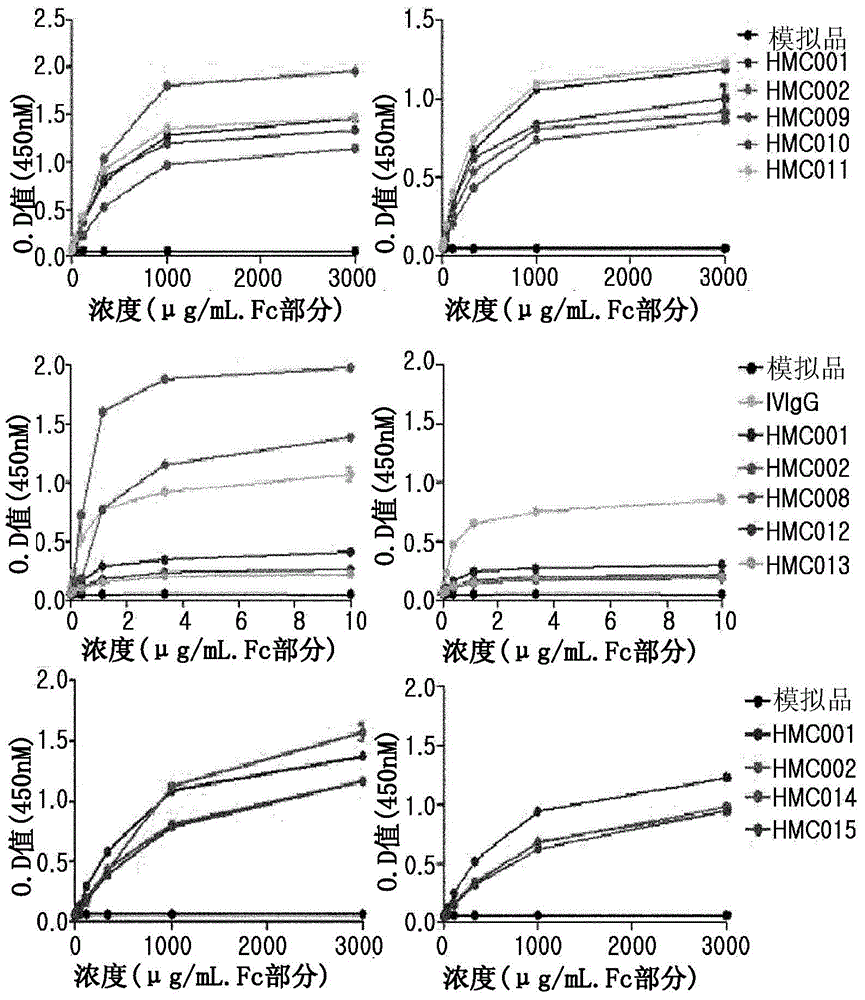
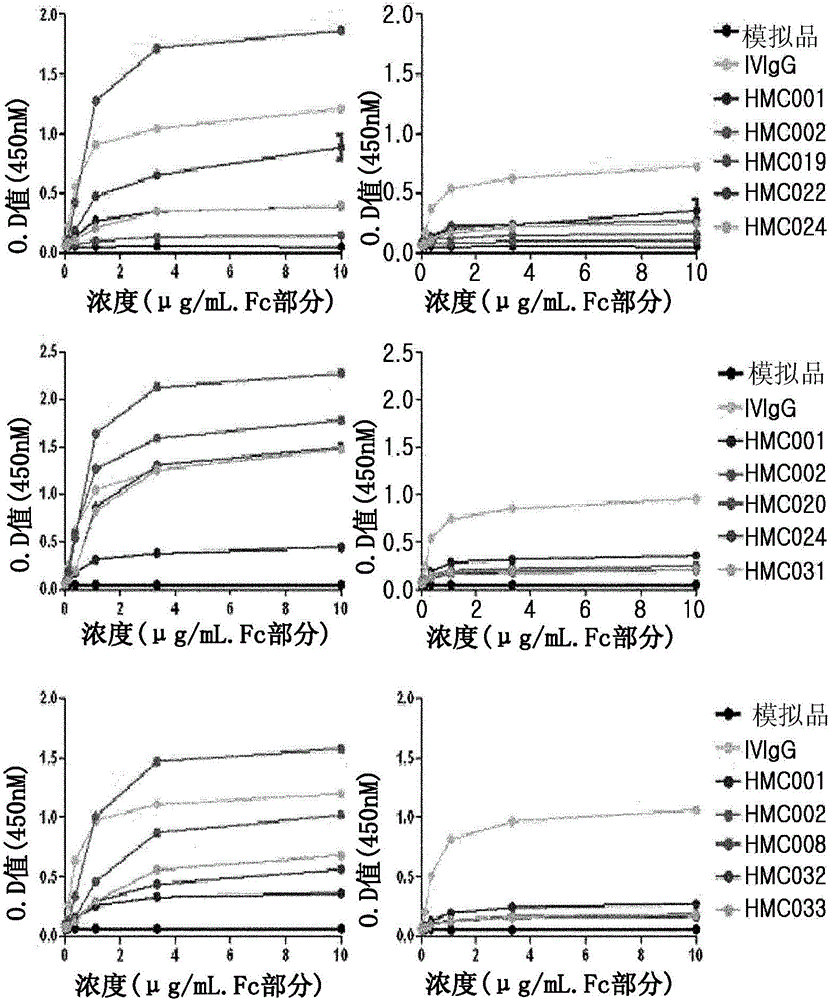
[0065] A position involved in the binding affinity of human FcRn was selected from the amino acid sequence (SEQ ID NO: 73) of HMC001 produced by Escherichia coli transformant HM11201 (KCCM-10660P, Korean Patent No. 10-0824505), and mutations were induced thereon to An amino acid at a corresponding position is replaced with another amino acid in order to increase FcRn binding affinity. To achieve this, use nucleotide-substituted primers and the QuikChange TM Site-directed mutagenesis kit (Stratagene). In this regard, primers used for site-directed mutagenesis are shown in Tables 1 to 3 below.
[0066] For site-directed mutagenesis by polymerase chain reaction (PCR), 50 ng of HMC001 expression vector, each primer pair, dNTP and PfuTurbo TM Polymerase (Stratagene) was added to the PCR tube and the reaction was performed as follows: 16 cycles of denaturation at 95°C for 30 seconds, 30 seconds a...
Embodiment 2
[0081] Example 2: Production of immunoglobulin Fc variants in E. coli
[0082]The expression vectors of immunoglobulin Fc variants prepared in Example 1 were respectively transformed into Escherichia coli BL21(DE3) competent cells (Invitrogen) by heat shock at 42°C for 1 minute, followed by solid culture in LB supplemented with ampicillin Colonies were cultured to select ampicillin-resistant colonies.
[0083] The colonies thus selected were inoculated on 500 ml of 2×LB medium (containing ampicillin) and cultured in a shaking incubator at 37° C. and 20 rpm for 15 hours. After transferring 100 ml of the culture broth to 500 ml of fresh 2×LB medium (containing ampicillin), the resulting solution was incubated until an optical density at 600 nm (OD600) of 4 to 5 was reached. Transfer 200ml of culture solution to a 5L-fermenter at a ratio of 10% (v / v), inject 2×LB medium (containing ampicillin) into it, and incubate at 37°C, 500-700rpm and 1vvm aeration rate A semi-automatic p...
Embodiment 3
[0084] Example 3: Isolation and purification of immunoglobulin Fc variants
[0085] The culture broth fermented in Example 2 was centrifuged at 12,000 g for 30 minutes to recover cell pellets. The cell pellet thus recovered was suspended in 10× volume of lysis buffer (20 mM Tris (pH 9.0), 1 mM EDTA (pH 8.0), 0.2 M NaCl, 0.5% triton X-100) and then heated at 15,000 psi Using a microfluidic machine (microfluidic chuck) under pressure three times. The cell lysates thus obtained were centrifuged at 6,000 g for 30 minutes to obtain only the inclusion bodies of the proteins expressed by the E. coli transformants.
[0086] Inclusion bodies were rinsed with 0.5% Triton X-100 and distilled water, and suspended in 8M urea solution containing 10× volume of 20 mM Tris (pH 9.0) for 2 hours to dissolve them. To separate undissolved solid impurities, the solution in which the inclusion bodies were dissolved was centrifuged at 12,000 g for 30 minutes to collect the supernatant. Then, L-c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com