New trifluoro methylation reagent and preparation and application thereof
A technology of trifluoromethylation and trifluoromethyl metal, applied in the direction of introducing hydroxyl and halogen preparation, sulfonic acid preparation, halogenated hydrocarbon preparation, etc., can solve the problems of difficult preparation, high equipment requirements, long process route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
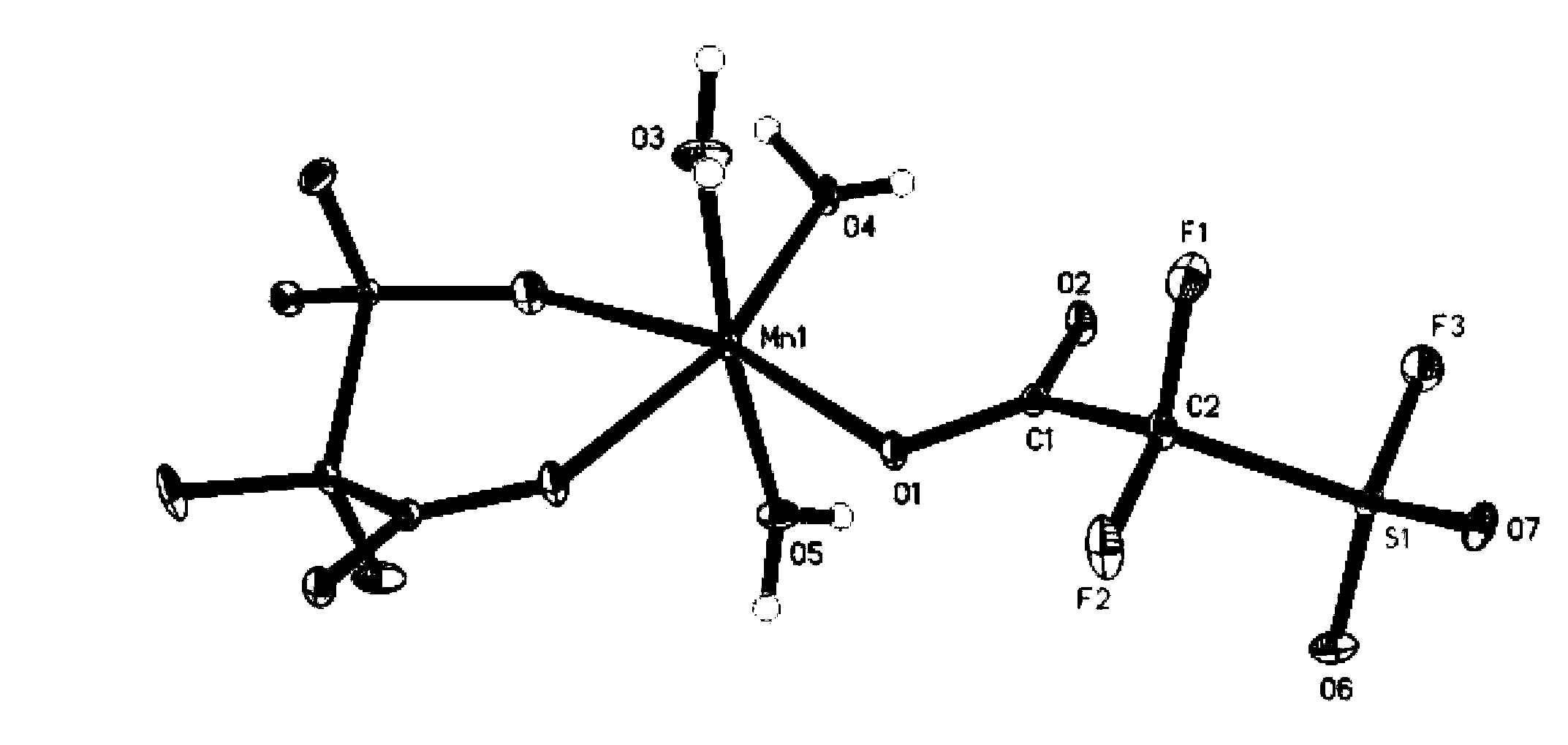
Image
Examples
preparation example Construction
[0132] The raw materials of the preparation method are readily available, and the compound of the formula II can be prepared by any prior art or obtained through commercial channels. Preferably, the compound of the formula II can use common fluorine chemical raw materials.
[0133] The method has a very high yield, generally ≥80%, and in another preferred embodiment, the yield of the reaction is ≥90%.
[0134] In a preferred embodiment of the present invention, described method comprises the following steps:
[0135] 1) Add the compound of formula III into the reactor, add an inert solvent and mix well;
[0136] 2) Add the compound of formula II dropwise into the reaction system;
[0137] 3) Stir overnight at -20-50°C;
[0138] 4) Filtrate and collect the filtrate, spin the solvent to obtain the compound of formula I;
[0139] In the above formulas, the definitions of each group are as described above.
[0140] The fluorosulfone difluoroacetic acid metal salt in acetonitri...
Embodiment 1
[0168] Embodiment 1 Fluorosulfone base silver difluoroacetate ((FSO 2 CF 2 COO) 2 Ag) Preparation
[0169] In a 500ml three-necked bottle equipped with electromagnetic stirring, constant pressure dropping funnel and reflux condenser, add silver carbonate Ag 2 CO 3 (100g, 0.363mol) and ether (200ml), add fluorosulfone difluoroacetic acid (FSO 2 CF 2 COOH, 129g, 0.725mol), stirred overnight. After filtration, wash the filter residue three times with a little ether, combine the ether layers, and remove the ether under reduced pressure to obtain white solid fluorosulfone silver difluoroacetate (FSO 2 CF 2 COOAg), yield 90%.
[0170] 19 F NMR (CDCl 3 ):40ppm(1F),-103ppm(2F)
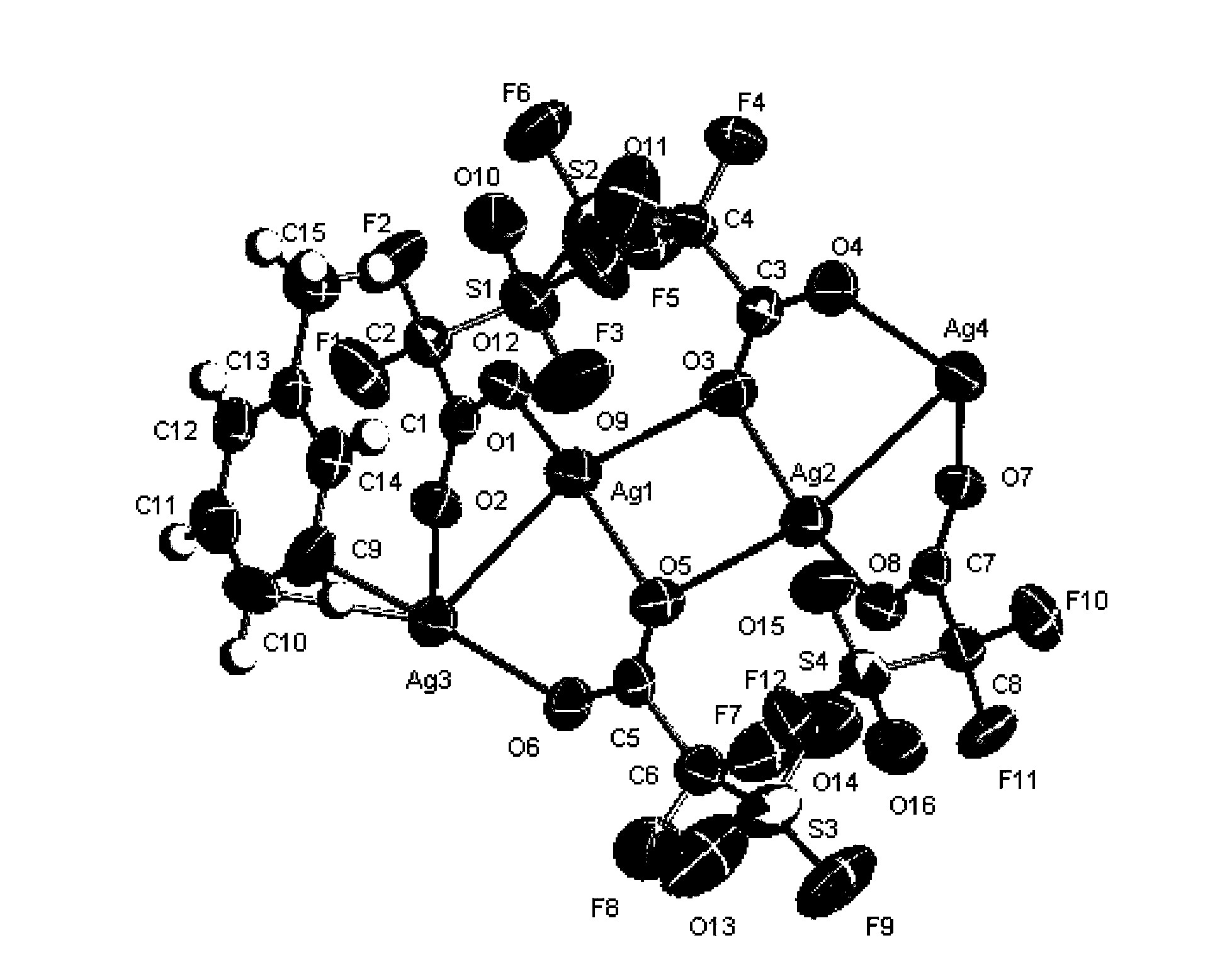
[0171] The white solid (FSO 2 CF 2 COOAg) was recrystallized in toluene, and the obtained crystal structure was as attached figure 1 shown.
Embodiment 2
[0172] Embodiment 2 fluorosulfone base copper difluoroacetate ((FSO 2 CF 2 COO) 2 Cu) Preparation
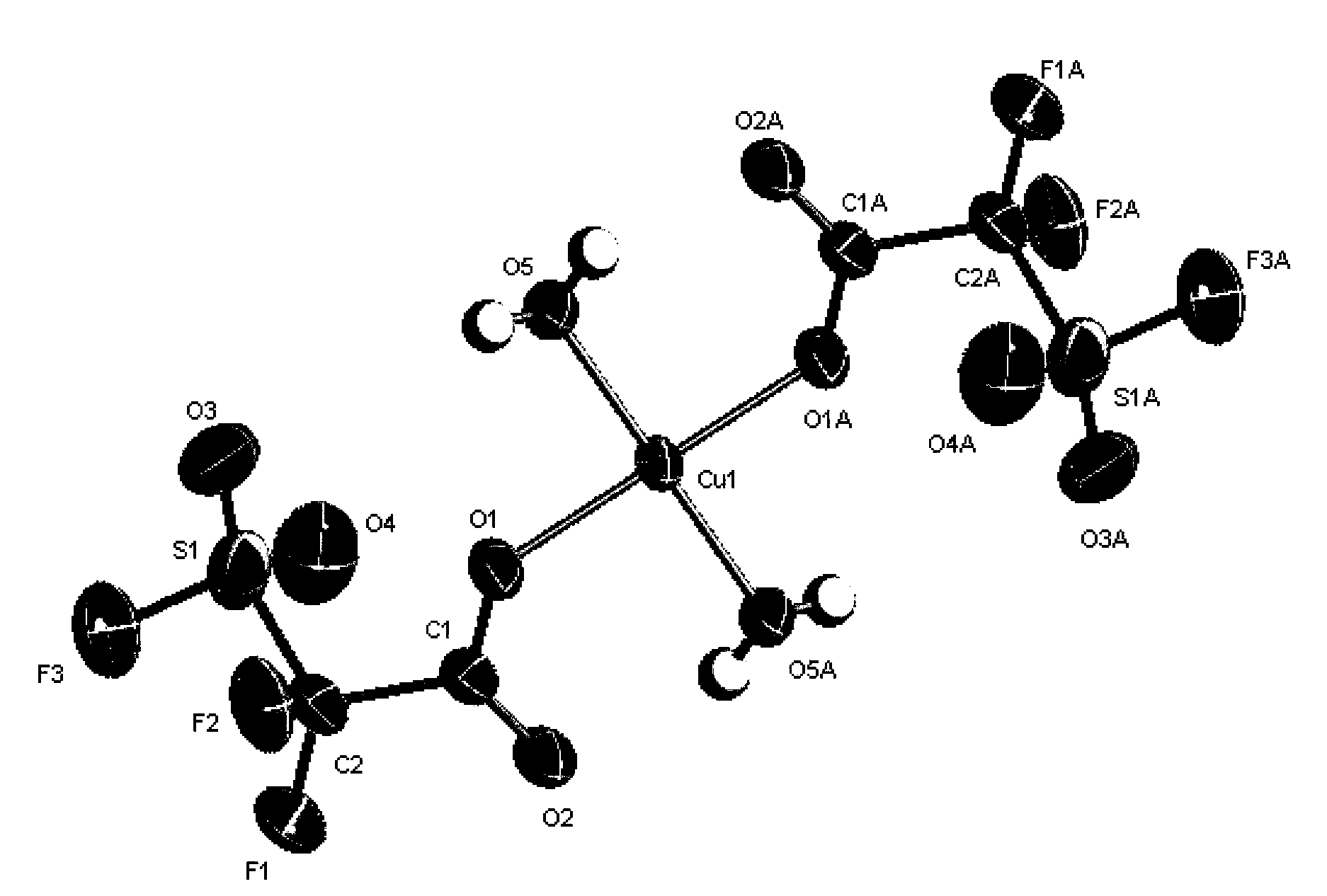
[0173] In a 500ml three-necked bottle equipped with electromagnetic stirring, constant pressure dropping funnel and reflux condenser, add basic copper carbonate Cu 2 (OH) 2 CO 3 (50g, 0.226mol) and ether (200ml), add fluorosulfone difluoroacetic acid (FSO 2 CF 2 COOH, 160g, 0.904mol), stirred overnight. After filtering, wash the filter residue three times with a little ether, combine the ether layers, and remove the ether under reduced pressure to obtain a blue-green solid fluorosulfone group copper difluoroacetate ((FSO 2 CF 2 COO) 2 Cu), yield 95%. The crystal structure of the product is attached figure 2 shown.
[0174] 19 F NMR (CDCl 3 ): 40ppm (1F), -103ppm (2F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com