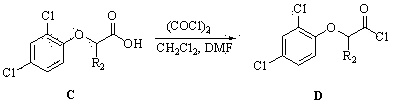6-aryloxy acetoxy aurone compound and application thereof on pesticide
A kind of technology of aryloxyacetoxy aurone and compound, which is applied in the field of herbicides, can solve the problems that 6-aryloxy acetoxy arurone compounds have not been disclosed, and achieve high herbicidal activity, high control effect and herbicidal activity Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: 2,4-dihydroxy- a - Chloroacetophenone ( A ) preparation:
[0036] Dissolve 20.0 g (181.6 mmol) of anhydrous resorcinol and 13.0 mL (205.0 mmol) of chloroacetonitrile in 300 mL of dry diethyl ether, cool the reaction flask to below 0 °C with an ice-salt bath, stir, and add 12.0 g ( 88.0 mmol) to dry molten zinc chloride, continue to feed dry hydrogen chloride gas for 3 h, then seal the reaction bottle, place it in the refrigerator for 1 d, the bottom layer is a red oily substance; continue to feed dry hydrogen chloride gas for 4 h, and then Placed in the refrigerator for 1 day, the bottom layer precipitated as a light yellow solid. Filter, wash the filter cake twice with anhydrous ether, then transfer it to a round bottom flask, hydrolyze it with 250 mL of water at a temperature lower than 100 °C until it turns transparent yellow-brown, put it in the refrigerator for 3 h, and precipitate yellow-brown The solid was filtered, dried, and recrystallized from et...
Embodiment 2
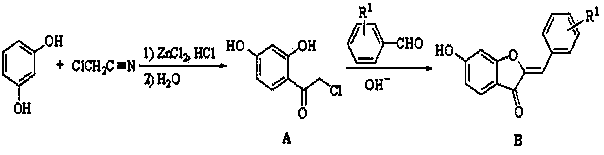
[0038] Embodiment 2: 6-hydroxyl-4 '-dimethylamino aurone ( B1 ) preparation:
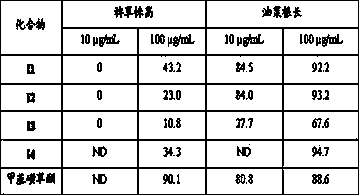
[0039] 2.0 g (10.7 mmol) A Add to a round-bottomed flask filled with ethanol (5 mL) and newly prepared 10% KOH solution (15 mL), then add 2.24 g (15.0 mmol) 4-dimethylaminobenzaldehyde, stir at room temperature, and after the reaction is complete , acidified with 1.0 mol / L dilute hydrochloric acid, allowed to stand for 1-3 h, filtered with suction, dried, and recrystallized with dichloromethane and ethanol to obtain 6-hydroxy-4′-dimethylaminoaurone ( B1 ) 1.86 g, yield 61.8%, m.p. 178~180 °C.
Embodiment 3
[0040] Embodiment 3: 6-hydroxyl-3 '-fluoro orange ketone ( B2 ) preparation:
[0041] 2.0 g (10.7 mmol) A Add it to a round-bottomed flask filled with ethanol (5 mL) and newly prepared 10% KOH solution (15 mL), then add 1.86 g (15.0 mmol) m-fluorobenzaldehyde, stir at room temperature, and after the reaction is complete, use 1.0 mol / L dilute hydrochloric acid for acidification, standing for 1-3 h, suction filtration, drying, and recrystallization with dichloromethane and ethanol to obtain 6-hydroxy-3'-fluoroorangeone ( B2 ) 2.04 g, yield 74.4%, m.p. 272~274 °C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com