Corneal surface protectant as well as preparation method and application thereof
A corneal surface and protective agent technology, applied in the field of medical supplies, to achieve good antibacterial and anti-infection effects, speed up the progress of surgery, and small changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] In cataract extraction + intraocular lens implantation, sodium hyaluronate with a molecular weight of 500,000 is used at a concentration of 9 mg / ml.
[0062] The production method is: weigh 0.045g of NaH 2 PO 4 ·H 2 O, 0.2g of Na 2 HPO 4 , and 0.44g of NaCl, dissolved in 100mL water for injection to prepare buffer solution A, add tobramycin powder to the buffer solution to a final concentration of 3mg / ml, and dexamethasone stock solution to a final concentration of 0.6mg / ml, and stir evenly , until the above-mentioned raw materials are completely dissolved, buffer solution B is obtained, and the buffer solution B is sterilized by filtration with a 0.22 micron filter membrane; 0.9 g of sodium hyaluronate with a molecular weight of 500,000 is dissolved in 100 ml of buffer solution to obtain cornea Protective agent;
[0063] Use a syringe to drop 0.2ml of the product of the present invention on the surface of the cornea, and form a flat liquid interface after 2 second...
Embodiment 2
[0065] Use the method in Example 1 to make a buffer solution, and dissolve 1.5 g of chondroitin sulfate with a molecular weight of 20,000 in 100 ml of buffer solution to obtain a corneal protective agent, which is used in cataract extraction+intraocular lens implantation;
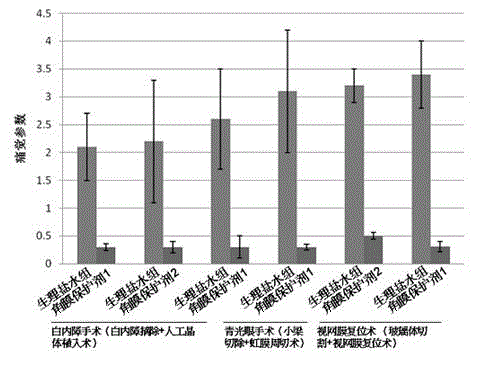
[0066] Use a syringe to drop 0.2ml of the product of the present invention on the surface of the cornea. After 2 seconds, a liquid interface with a flat surface will be formed. The maintained good refractive state and flat surface will last for about 10 minutes. 1 time. A clinical investigation was carried out on the postoperative effects of 16 patients, including 10 in the normal saline group and 6 in the corneal protective agent group. T test was used to compare the statistical differences between the two experimental groups, and P image 3 ) (P Figure 4 ) (P<0.05).
Embodiment 3
[0068] Use the method in embodiment 1 to make buffer, molecular weight is that 700,000 chitosan 1.0g is dissolved in the buffer of 100ml, obtains corneal protectant, is used in cataract extraction+intraocular lens implantation;
[0069] Use a syringe to drop 0.2ml of the product of the present invention on the surface of the cornea. After 2 seconds, a liquid interface with a flat surface will be formed. The maintained good refractive state and flat surface will last for about 10 minutes. 1 time. A clinical investigation was conducted on the postoperative effects of 13 patients, including 5 in the normal saline group and 8 in the corneal protective agent group. T test was used to compare the statistical differences between the two experimental groups, and P image 3 ) (P Figure 4 ) (P<0.05).
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com