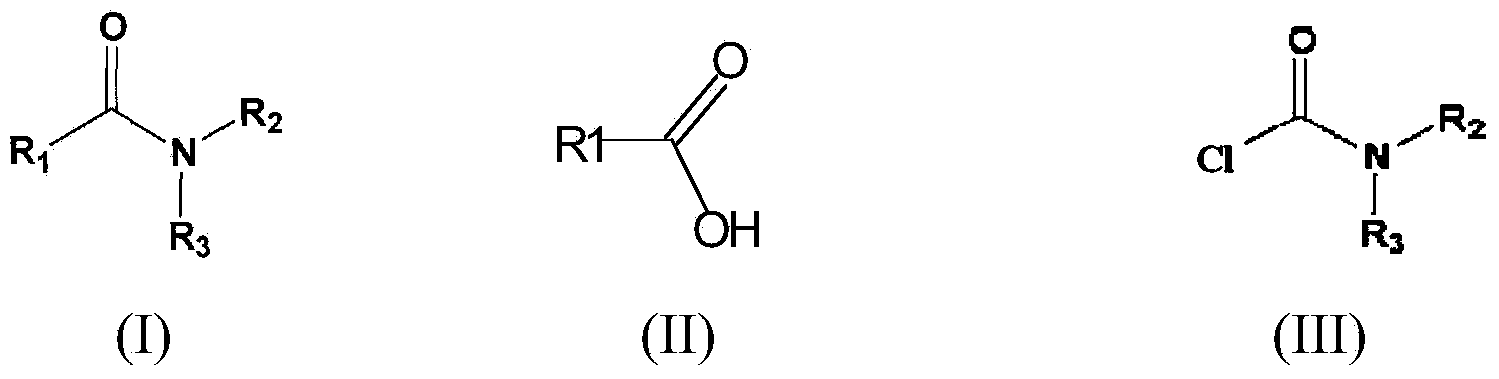
Process for preparation of n,n-di substituted carboxamides
A disubstituted, carboxamide compound technology, applied in the direction of carboxylic acid amide preparation, organic compound preparation, chemical instruments and methods, etc., can solve problems such as difficult to apply on an industrial scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
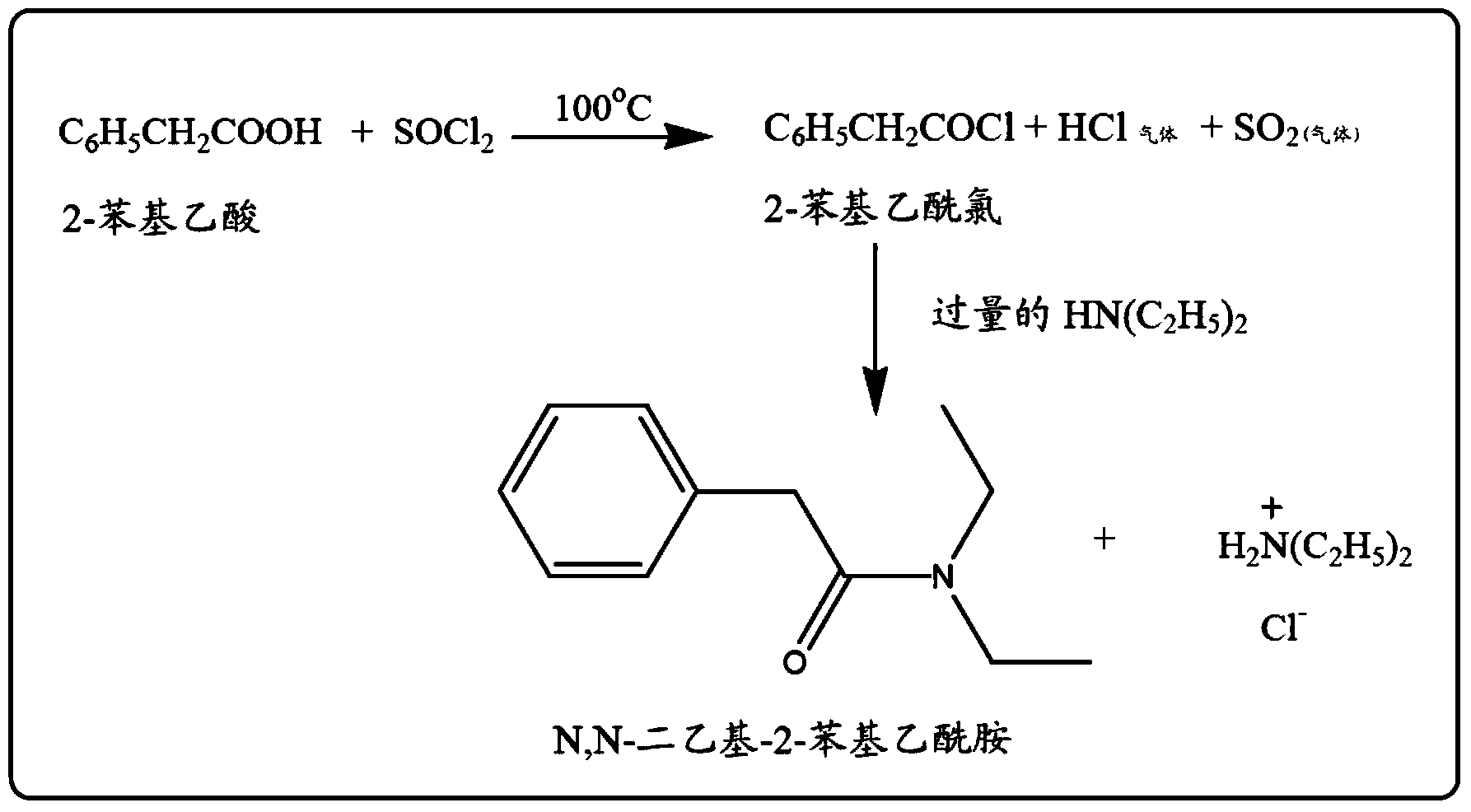
[0096] Preparation of N,N-diethyl-2-phenylacetamide (DEPA)
[0097] 136 g (1 mole) of phenylacetic acid and 136 g (=127 ml, 1 mole) of N,N-diethylcarbamoyl chloride were placed in a 1-liter two-neck round bottom flask equipped with an air condenser placed on a magnetic stirrer. Add 98 g (96 ml, 1.2 moles) of 1-methylimidazole (an organic tertiary base) to the above 1 L two-neck round bottom flask at room temperature using a pressure equalizing funnel fitted into the side neck of the round bottom flask . After the addition was complete, the reaction mixture was stirred continuously for 30 minutes at room temperature. Then, the reaction mixture was treated with 250 ml of water and the two layers were separated. The pure and colorless product N,N-diethyl-2-phenylacetamide (DEPA) was obtained by vacuum distillation of the organic layer.
[0098] The purity of the compound was analyzed using GC-MS and the purity of the compound was greater than 99.5%. And, the yield of product ...
Embodiment 2
[0101] Preparation of N,N-diethyl-m-toluamide (DEET)
[0102] Put 136g (1 mole) of m-toluic acid (3-methylbenzoic acid) and 136g (=127ml, 1 mole) of N,N-diethylcarbamoyl chloride into a magnetic stirrer equipped with air condensing in a 1-liter two-necked round-bottom flask. At room temperature, 121 g (167 ml, 1.2 moles) of triethylamine (an organic tertiary base) was added to the above 1 liter two-neck round bottom flask using a pressure equalizing funnel fitted in the side neck of the round bottom flask. After the addition was complete, the reaction mixture was stirred continuously at room temperature for 20 minutes. Then, the reaction mixture was treated with 250 ml of water and the two layers were separated. The pure and colorless product N,N-diethyl-m-toluamide (DEET) was obtained by vacuum distillation of the organic layer.
[0103] The purity of the compound was analyzed using GC-MS and the purity of the compound was greater than 99.5%. The yield of the product was ...
Embodiment 3
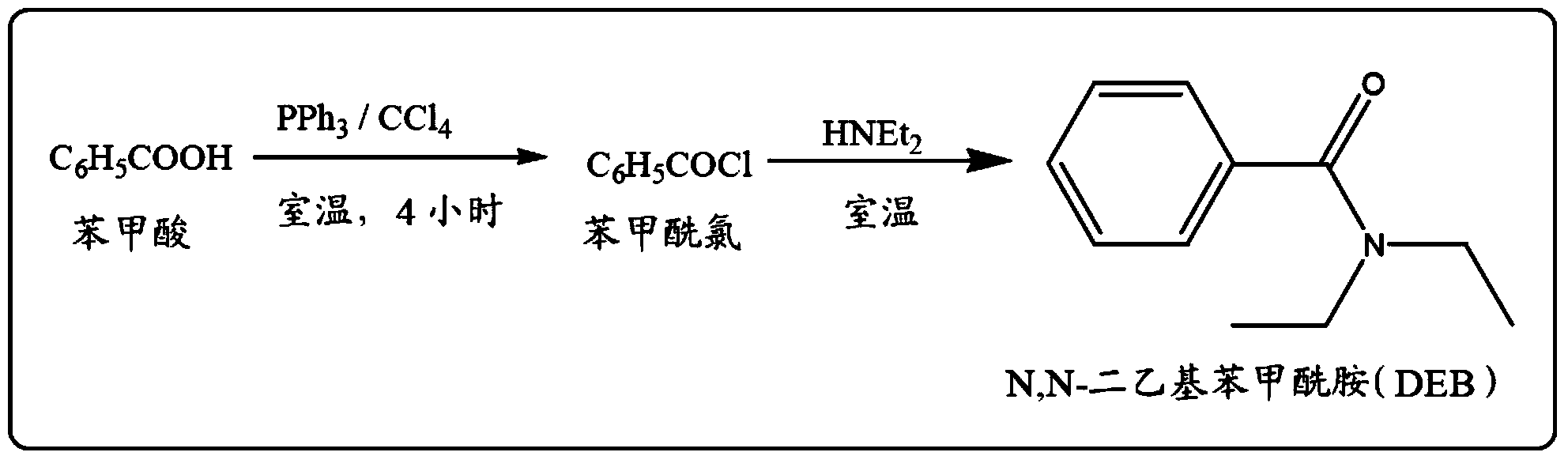
[0106] Preparation of N,N-diethylbenzamide (DEB)
[0107] 122 g (1 mole) of benzoic acid and 136 g (=127 ml, 1 mole) of N,N-diethylcarbamoyl chloride were placed in a 1-liter two-neck round bottom flask equipped with an air condenser placed on a magnetic stirrer. At room temperature, 222 g (285 ml, 1.2 moles) of tributylamine was added to the above-mentioned 1-liter two-necked round-bottomed flask using an equalizing funnel fitted in the side neck of the round-bottomed flask. After the addition was complete, the reaction mixture was stirred continuously at room temperature for 20 minutes. Then, the reaction mixture was treated with 250 ml of water and the two layers were separated. The pure and colorless product N,N-diethylbenzamide (DEB) was obtained by vacuum distillation of the organic layer.
[0108] The purity of the compound was analyzed using GC-MS and the purity of the compound was greater than 99.5%. The yield of the product was 173 g (98%).
[0109]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com