Amorphous substance of canagliflozin and preparation method of amorphous substance
An amorphous, endothermic peak technology, applied in the field of medicine, can solve the problems of high industrialization cost and poor control, and achieve the effects of simple preparation method, good stability, and good preparation adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of Canagliflozin Amorphous Form
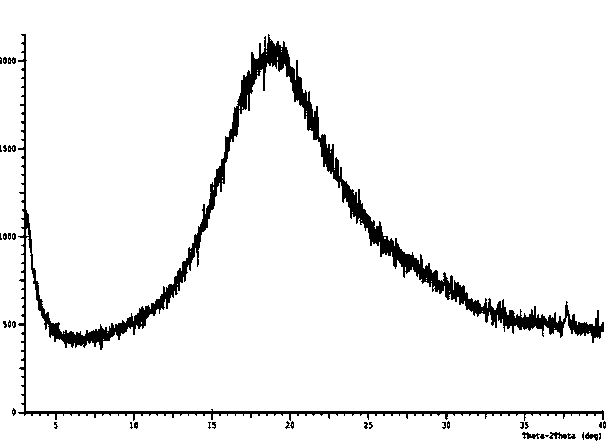
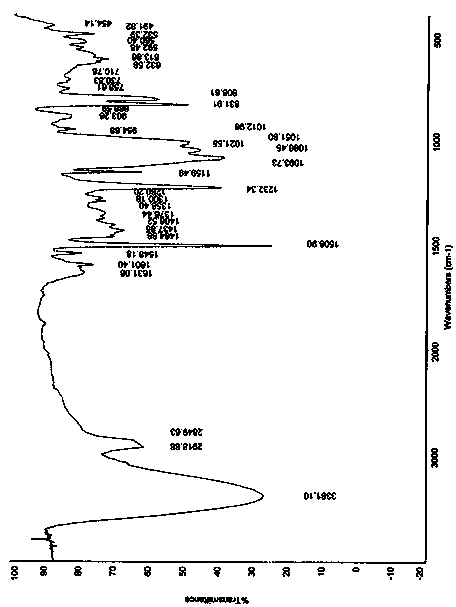
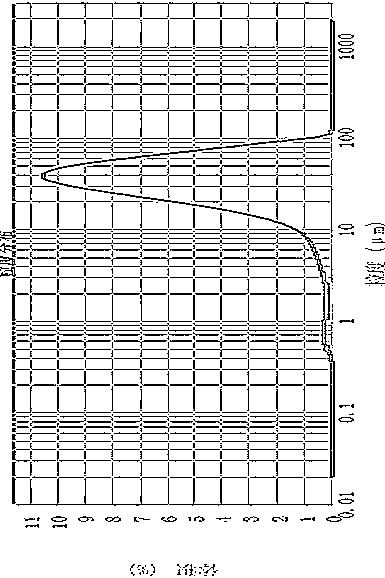
[0057] Dissolve 20g of canagliflozin in 80ml of ethyl acetate at 50-60°C, add dropwise to 160ml of cyclohexane solution at room temperature, stir while adding dropwise, continue stirring for 1 hour at room temperature after the dropwise addition, precipitate solid, and filter; The filter cake was dried at 30-40°C to obtain 18 g of amorphous canagliflozin, HPLC: 99.5%. Test X-ray powder diffraction, the results are as follows figure 1 ;Test TG-DSC, the result is as follows figure 2 ;Test the infrared spectrum, the result is as follows image 3 ;Test granularity, the result is as follows Figure 4 .
Embodiment 2
[0058] Embodiment 2 (comparative embodiment)
[0059] Preparation of Canagliflozin Amorphous Form
[0060] Dissolve 10g of canagliflozin in 100ml of ethyl acetate at 30-40°C, quickly distill off the solvent under reduced pressure at 40-50°C to obtain a solid powder; dry under reduced pressure at 40-50°C to obtain 9.9g of canagliflozin amorphous, HPLC: 99.5%. Test X-ray powder diffraction, the results are as follows Figure 5 ;Test TG-DSC, the result is as follows Image 6 .
[0061]
Embodiment 3
[0063] Preparation of Canagliflozin Amorphous Form
[0064] Dissolve 50g of canagliflozin in 300ml of toluene at 20-30°C, add dropwise to 150ml of n-hexane solution with an internal temperature of 0-10°C under stirring, continue to stir for 0.5h at 0-10°C after the dropwise addition, and a solid precipitates , filtered; the filter cake was dried under reduced pressure at 40-50° C. to obtain 43 g of canagliflozin amorphous, HPLC: 99.7%.
[0065] The resulting amorphous has Figure 1-3 Characteristics.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com