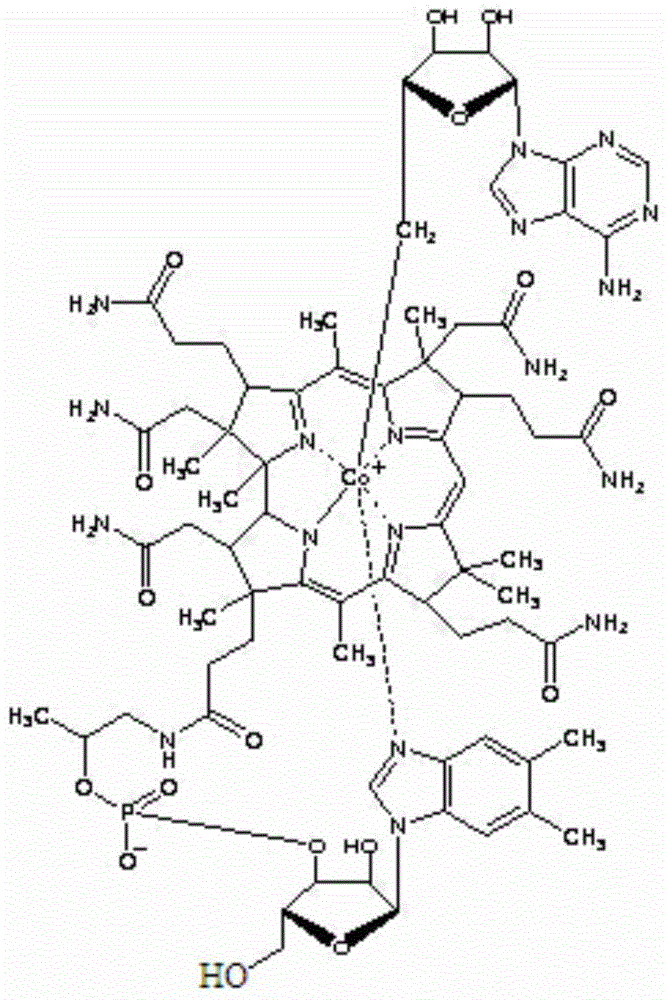
A kind of adenosylcobalamin compound and its pharmaceutical composition
A technology of adenosylcobalamin and composition, which is applied in the field of pharmaceutical compositions containing the adenosylcobalamin, which can solve the problems of uneven and fine sample appearance, poor preparation stability, complex process, etc., and achieve uniform and fine appearance , improved stability and good resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of adenosylcobalamin compound:
[0054] (1) Add 50 g of adenosylcobalamin solid into 150 mL of a mixed solvent of isopropanol and pyridine, the volume ratio of isopropanol and pyridine in the mixed solvent is 4.0:1.0, and dissolve the adenosylcobalamin solid at room temperature;
[0055] (2) Add 800mL ether to the solution obtained in step (1), while stirring, the stirring rate is controlled at 1600r / min;
[0056] (3) After adding diethyl ether, cool down to -10°C within 5 minutes, and stand at -10°C for 11 hours to precipitate crystals, filter, and wash the filter cake twice with ether (150mL each time). After drying under vacuum for 4 hours, adenosylcobalamin crystalline compound was obtained.
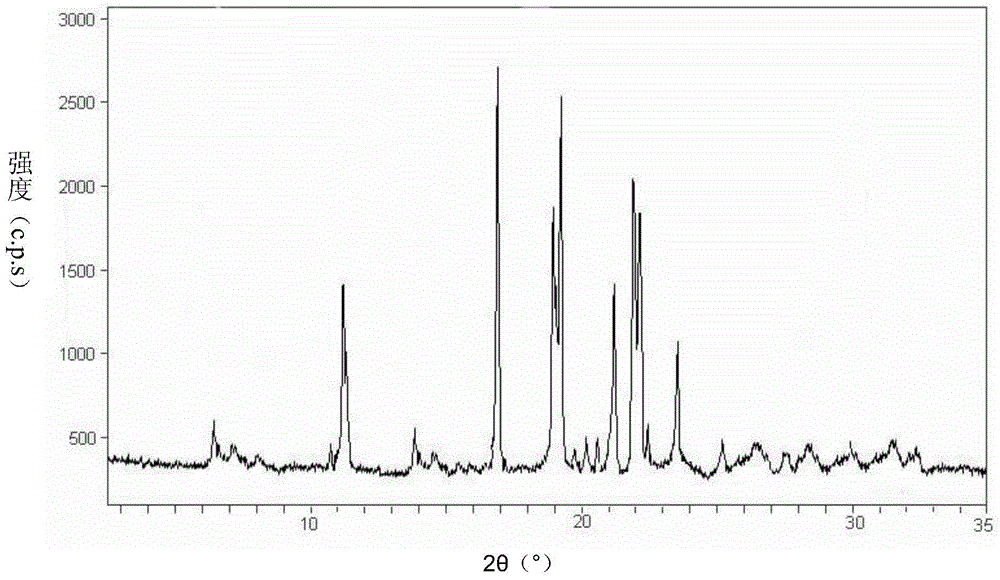
[0057] The adenosylcobalamin compound uses Cu-Kα 1 X-ray powder diffraction for radiographic measurements such as figure 1 shown.
Embodiment 2
[0059] Preparation of adenosylcobalamin compound:
[0060] (1) Add 50 g of adenosylcobalamin solid into 150 mL of a mixed solvent of isopropanol and pyridine, the volume ratio of isopropanol and pyridine in the mixed solvent is 5.0:1.0, and dissolve the adenosylcobalamin solid at room temperature;
[0061] (2) Add 500mL ether to the solution obtained in step (1), while stirring, the stirring rate is controlled at 1800r / min;
[0062] (3) After adding ether, cool down to -15°C within 3 minutes, and let it stand at -15°C for 10 hours to precipitate crystals, filter, and wash the filter cake twice with ether (150mL each time). After drying under vacuum for 3 hours, adenosylcobalamin crystalline compound was obtained.
[0063] The adenosylcobalamin compound uses Cu-Kα 1 Radiographic X-ray powder diffraction showed that, with the attached figure 1 The results shown match.
Embodiment 3
[0065] Preparation of adenosylcobalamin compound:
[0066] (1) Add 50 g of adenosylcobalamin solid into 150 mL of a mixed solvent of isopropanol and pyridine, the volume ratio of isopropanol and pyridine in the mixed solvent is 2.0:1.0, and dissolve the adenosylcobalamin solid at room temperature;
[0067] (2) add 600mL ether in the solution that step (1) obtains, stir simultaneously, stirring rate is controlled at 1500r / min;
[0068] (3) After adding ether, cool down to -10°C within 6 minutes, and stand at -10°C for 10 hours to precipitate crystals, filter, and wash the filter cake twice with ether (150mL each time), at a temperature of 35°C After drying under vacuum for 2 hours, adenosylcobalamin crystalline compound was obtained.
[0069] The adenosylcobalamin compound uses Cu-Kα 1 Radiographic X-ray powder diffraction showed that, with the attached figure 1 The results shown match.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com