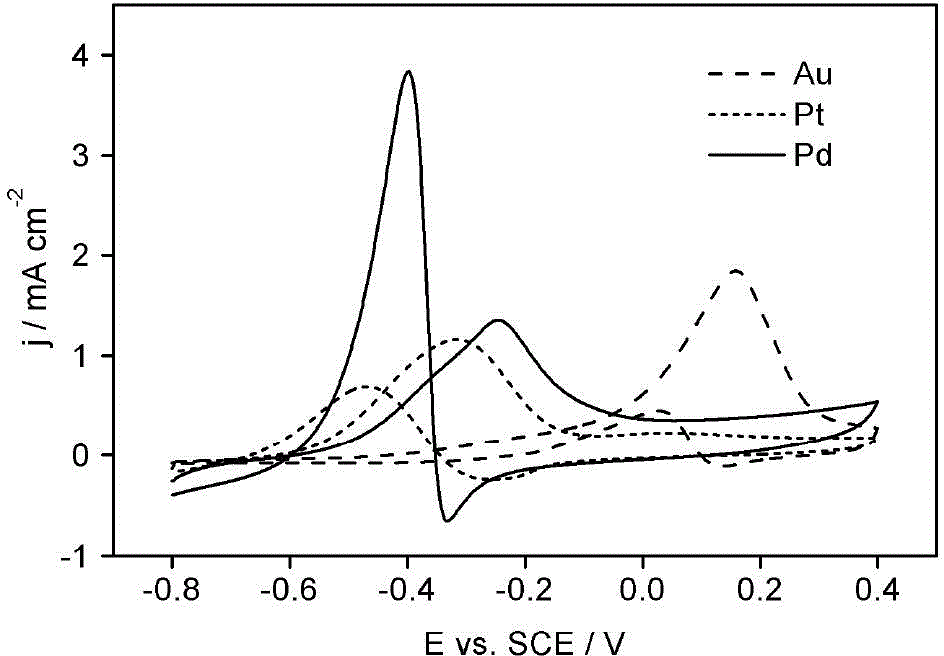
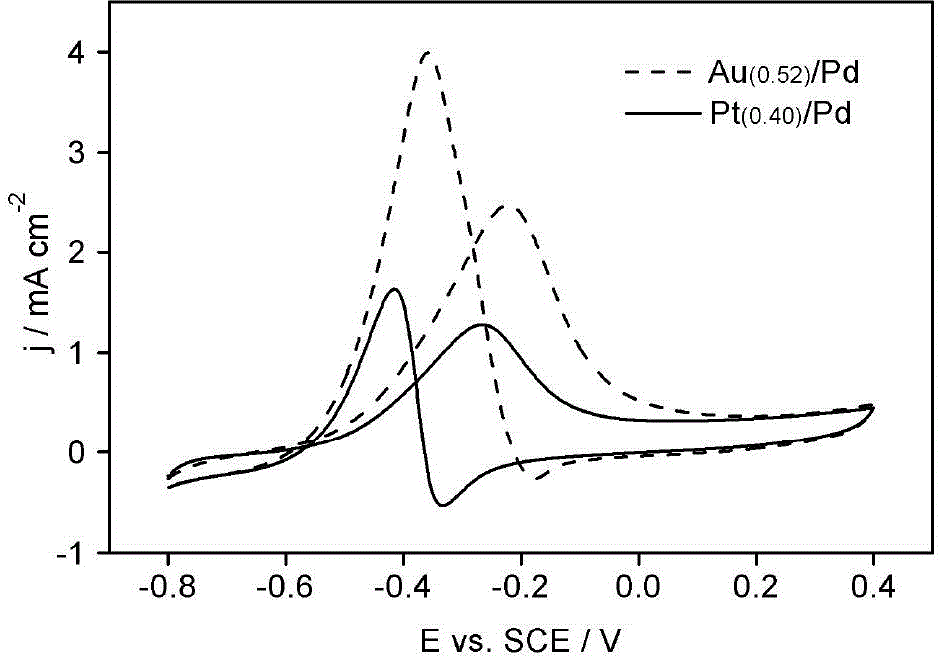
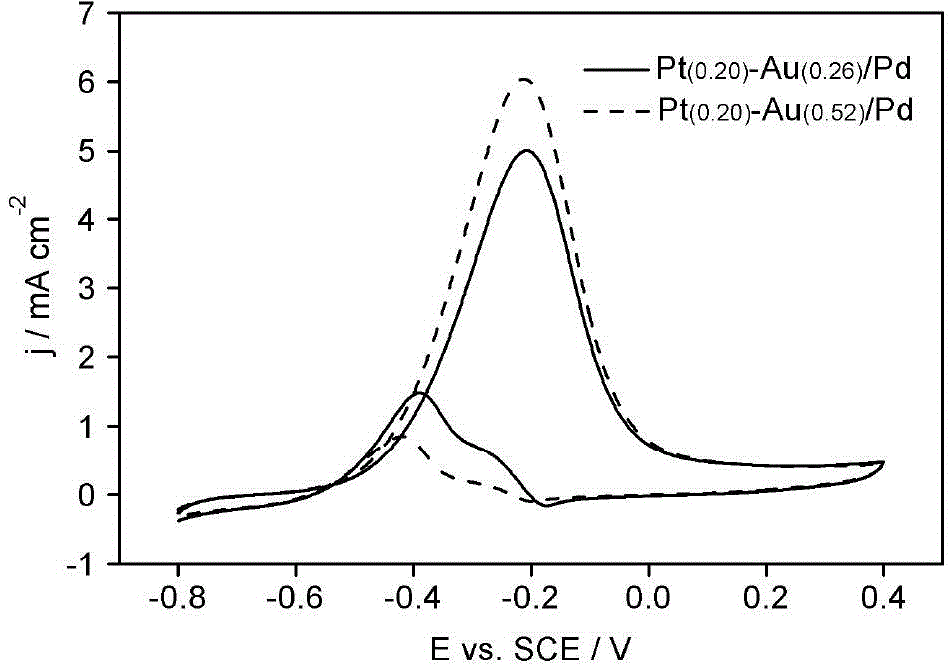
Electrode bimetal modification method
A metal modification, electrode technology, applied in the direction of material electrochemical variables, etc., can solve the problems of poor catalytic activity improvement effect, limited improvement of Pd electrode activity, low reaction activity, etc., to achieve electrode stability, simple modification method, and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Take 20mL double distilled water, 200μL 0.1mol·dm –3 Chlorauric acid and 53.2 μL of concentrated sulfuric acid (purity 98.0%) were added to a dry and clean 50 mL beaker to prepare a gold precursor solution containing 1 mmol dm –3 Chlorauric acid and 0.05mol dm –3 sulfuric acid.
[0026] (2) Take 20mL double distilled water, 200μL 0.1mol·dm –3 Chloroplatinic acid and 53.2 μL concentrated sulfuric acid (purity 98.0%) were added to a dry and clean 50 mL beaker to prepare a platinum precursor solution containing 1 mmol dm –3 Chloroplatinic acid and 0.05mol dm –3 sulfuric acid.
[0027] (3) Take 20mL double-distilled water, 118μL ethanol (purity ≥ 99.7%) and 0.4g sodium hydroxide (purity ≥ 96.0%) to a dry and clean 50mL beaker to prepare alcohol solution, which contains 0.1mol·dm –3 Ethanol and 0.5mol·dm –3 sodium hydroxide.
[0028] (4) Nitrogen gas is passed into the three solutions prepared above to discharge the oxygen dissolved therein.
[0029] (5) The surf...
Embodiment 2
[0034] (1) Take 20mL double distilled water, 200μL 0.1mol·dm –3 Chlorauric acid and 53.2 μL of concentrated sulfuric acid (purity 98.0%) were added to a dry and clean 50 mL beaker to prepare a gold precursor solution containing 1 mmol dm –3 Chlorauric acid and 0.05mol dm –3 sulfuric acid.
[0035] (2) Take 20mL double distilled water, 200μL 0.1mol·dm –3 Chloroplatinic acid and 53.2 μL concentrated sulfuric acid (purity 98.0%) were added to a dry and clean 50 mL beaker to prepare a platinum precursor solution containing 1 mmol dm –3 Chloroplatinic acid and 0.05mol dm –3 sulfuric acid.
[0036] (3) Take 20mL double-distilled water, 118μL ethanol (purity ≥ 99.7%) and 0.4g sodium hydroxide (purity ≥ 96.0%) to a dry and clean 50mL beaker to prepare alcohol solution, which contains 0.1mol·dm –3 Ethanol and 0.5mol·dm –3 sodium hydroxide.
[0037] (4) Nitrogen gas is passed into the three solutions prepared above to discharge the oxygen dissolved therein.
[0038] (5) The surf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com