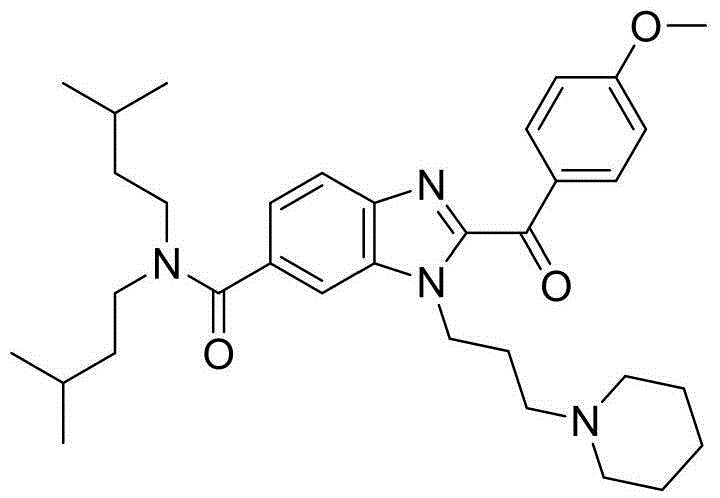
Application of Ipsen17 in preparing medicaments for treating obesity caused by melanocortin-4 receptor
A technology of corticosteroids and mutants, applied in the field of medicine, can solve problems such as functional defects and abnormal delivery of mutants, and achieve the effect of good cell membrane expression ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The construction of human melanocortin receptor-4 and its mutant expression vector of the present invention, the method is as follows:
[0032] According to the DNA sequence of the known human melanocortin receptor-4 gene (NCBI reference sequence: NM_005912.2), design primers and amplify its gene sequence, introduce restriction sites through PCR primers, and add EcoRI restriction upstream of the gene Restriction enzyme cutting site and HA marker sequence, XbaI restriction enzyme cutting site was added downstream, the nucleotide sequence generated by amplification was named EcoRI-HA-MC4R-XbaI, and it was cloned into the commercial vector pcDNA3.1(+) Among them, the insertion sites are EcoRI and XbaI, and the constructed vector containing this sequence is named pcDNA3.1-hMC4R. The primers were synthesized in Shanghai Jierui Bioengineering Co., Ltd. The reagents used for PCR amplification, the endonuclease and DpnⅠ enzyme used in the experiment were all Fermentas brand.
...
Embodiment 2
[0063] Expression of melanocortin receptor-4 and its mutants HEK293 cells were selected as hosts, transfection was performed by calcium phosphate transfection method, and the medium containing G418 was used for screening. The formulation of 0.1% Gelatin (Sigma) in the coating solution is as follows: 1 g of Gelatin was dissolved in 1 L of PBS, sterilized at high temperature, and stored at 4°C. The transfection reagent recipe is as follows: 2.5M CaCl 2 [18.38gCaCl 2 2H 2 O (Sigma) was dissolved in 30mL three-distilled water, passed through a 0.22μm Milipore filter membrane], 2×BSS (280mM NaCl, 1.5mM NaCl 2 HPO 3 , 50mM BES (Sigma), pH6.95, passed through a 0.22μm filter), stored at -20°C. The growth medium formulation was as follows: 50 mL fetal bovine serum, 500 μl gentamicin (40 mg / mL, Solarbio), 2.5 mL HEPES (1M, Sigma), 447 mL DMEM (high glucose, Gibco), stored at 4°C. The selection medium formula is as follows: 50 mL fetal bovine serum, 500 μl gentamicin (40 mg / mL), 5 ...
Embodiment 3
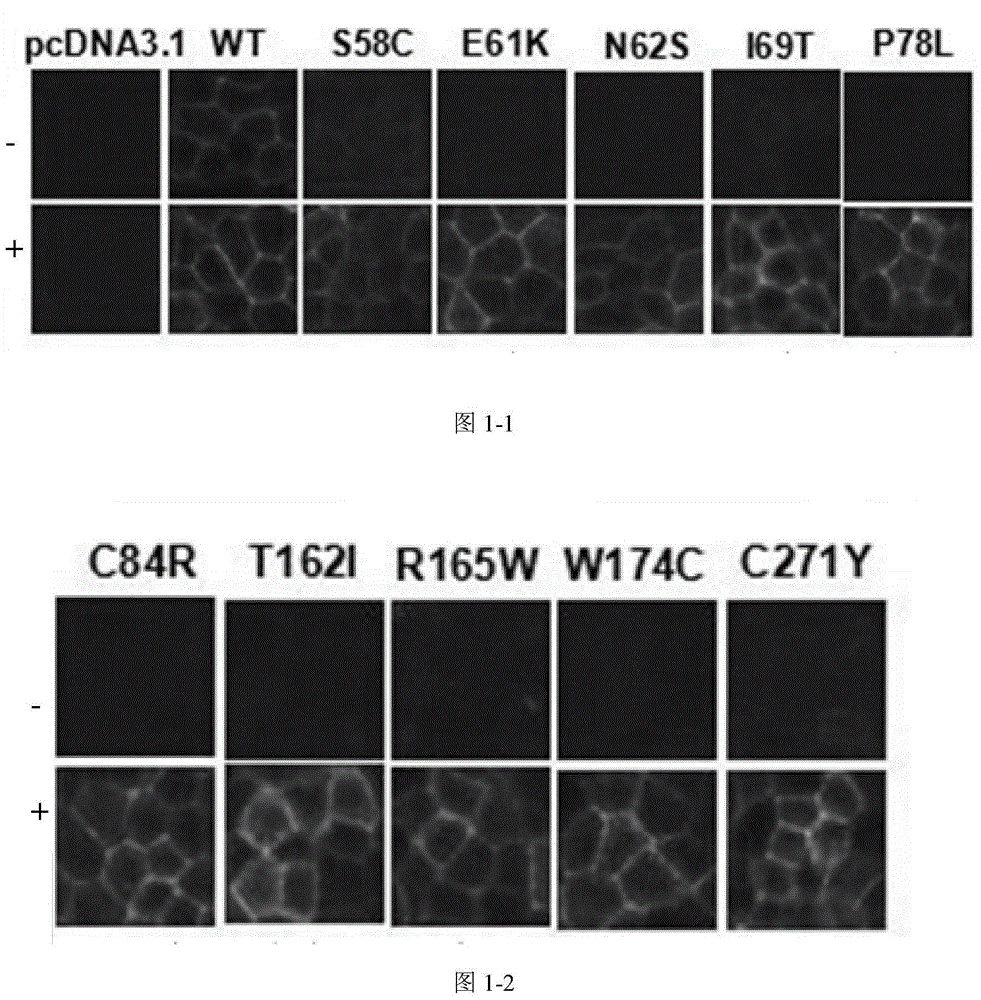
[0071] Quantitative analysis and determination of cell membrane expression of wild-type WT and each mutant expression vector in HEK293 was carried out by FACS. That is, to measure and analyze the difference in the expression level of the cell membrane of the constructed stable expression mixed cell line under the two conditions of treatment with the pharmaceutical chaperone molecule Ipsen17 and without treatment. The purity of Ipsen17 used in the experiment was 98%, which was synthesized by the applicant; the purity of α-MSH was 95%, and it was purchased from Shanghai Qiangyao Biotechnology Co., Ltd. The entire experimental operation after the culture is completed is carried out on ice, and the specific steps are as follows S1-S5:
[0072] S1: WT, mutant receptors, and empty vector pcDNA3.1 stable cell lines at 1×10 4 The density of cells / well was spread in a six-well plate and cultured for 24 hours. WT or mutant hMC4R expressing cell lines were added with control buffer or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com