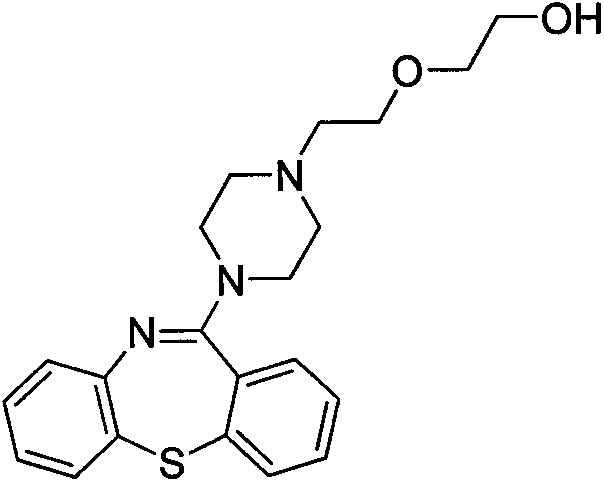
Preparation method for quetiapine intermediate
An intermediate, quetiapine technology, applied in the field of synthesis of chemical drug intermediates, can solve the problems of limiting industrial development prospects, environmental pollution, long production cycle, etc., to improve the reduction reaction yield, shorten the reaction time, and improve the reaction yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
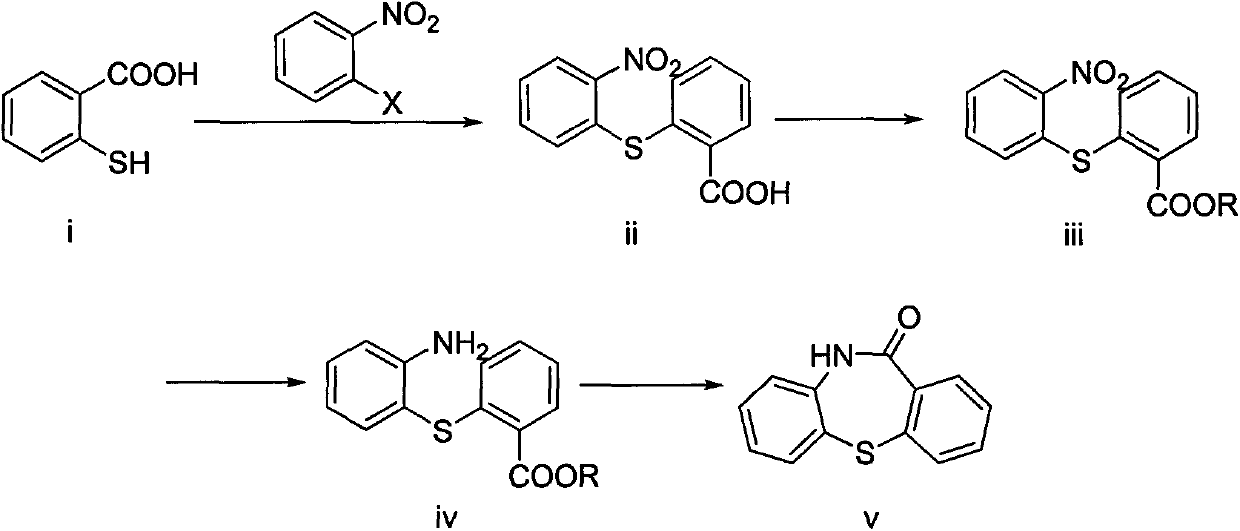
[0019] Add thiosalicylic acid (30.8 g, 0.2 mol) and 200 mL (2.5 mol / L) of aqueous sodium hydroxide solution into a 500 mL three-necked reaction flask, and stir. The temperature was raised to 40° C., o-bromonitrobenzene (44.4 g, 0.22 mol) was added, the temperature was raised to 100° C. and kept for 3 hours until the reaction was complete. The reaction solution was extracted with ethyl acetate to remove impurities, and the aqueous layer was acidified with hydrochloric acid until the pH was 1-2, and a large amount of yellow solid was precipitated to obtain 2-nitro-2'-carboxydiphenylsulfide (52.8 g, Yield 95.9%).
[0020] Put the prepared 2-nitro-2'-carboxydiphenylsulfide (52.8g, 0.19mol) into a 1000mL three-necked reaction flask, add 400mL of anhydrous methanol and stir to dissolve, add 5mL of concentrated sulfuric acid (98%) dropwise, drop After the addition was complete, the reaction was carried out at 60° C. for 8 hours to obtain the reaction liquid of (iii).
[0021] Add 2...
Embodiment 2
[0024] Add thiosalicylic acid (30.8 g, 0.2 mol) and 200 mL (2.5 mol / L) of potassium hydroxide aqueous solution into a 500 mL three-necked reaction flask, and stir. The temperature was raised to 40°C, o-fluoronitrobenzene (34.7 g, 0.22 mol) was added, the temperature was raised to 100°C and kept for 4 hours until the reaction was complete. The reaction solution was extracted with ethyl acetate to remove impurities, and the aqueous layer was acidified with hydrochloric acid until the pH was 2-3, and a large amount of yellow solid was precipitated to obtain 2-nitro-2'-carboxydiphenylsulfide (48.2 g, Yield 87.5%).
[0025] Put the prepared 2-nitro-2'-carboxydiphenylsulfide (48.2g, 0.17mol) into a 1000mL three-necked reaction flask, add 400mL of absolute ethanol and stir to dissolve, add 5mL of concentrated sulfuric acid (98%) dropwise, drop After the addition was complete, the reaction was carried out at 80° C. for 8 hours to obtain the reaction liquid of (iii).
[0026] Add 2.0...
Embodiment 3
[0029] Add thiosalicylic acid (30.8 g, 0.2 mol) and 200 mL (2.5 mol / L) of potassium hydroxide aqueous solution into a 500 mL three-necked reaction flask, and stir. The temperature was raised to 40°C, o-chloronitrobenzene (34.7 g, 0.22 mol) was added, the temperature was raised to 80°C and kept for 8 hours until the reaction was complete. The reaction solution was extracted with ethyl acetate to remove impurities, and the aqueous layer was acidified with hydrochloric acid until the pH was 1-2, and a large amount of yellow solid was precipitated to obtain 2-nitro-2'-carboxydiphenylsulfide (51.5 g, Yield 93.5%).
[0030] Put the prepared 2-nitro-2'-carboxydiphenylsulfide (51.5g, 0.19mol) into a 1000mL three-necked reaction flask, add 400mL of anhydrous methanol and stir to dissolve, add 5mL of concentrated sulfuric acid (98%) dropwise, drop After the addition was complete, the reaction was carried out at 65° C. for 8 hours to obtain the reaction liquid of (iii).
[0031] Add 2.0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com