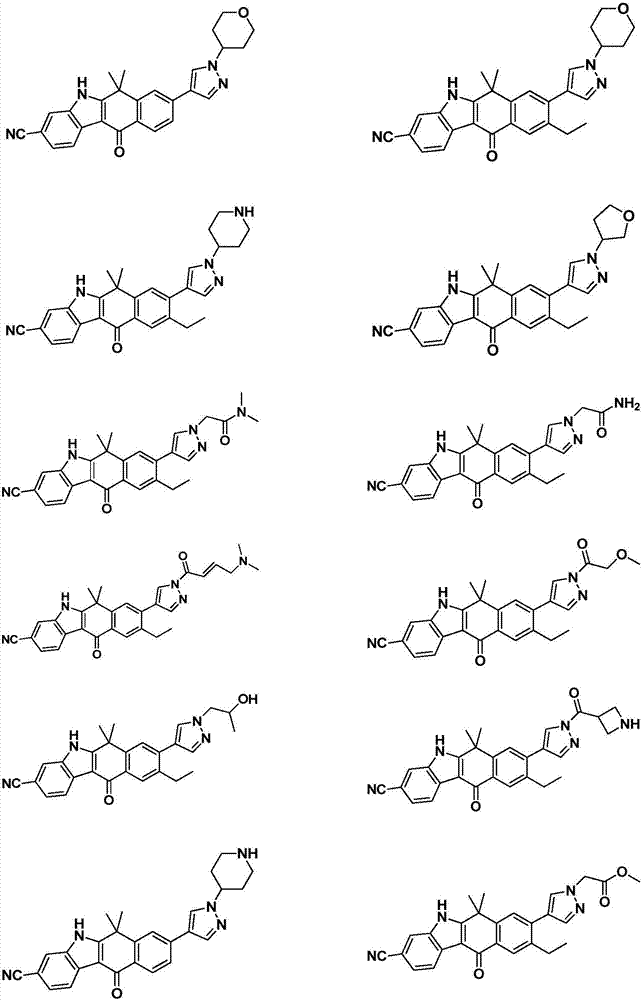
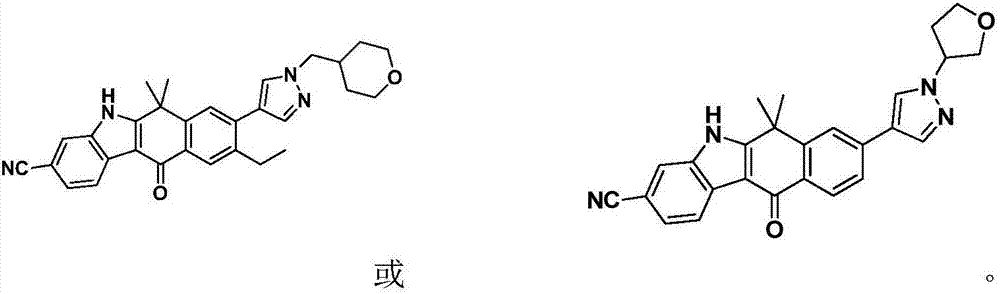
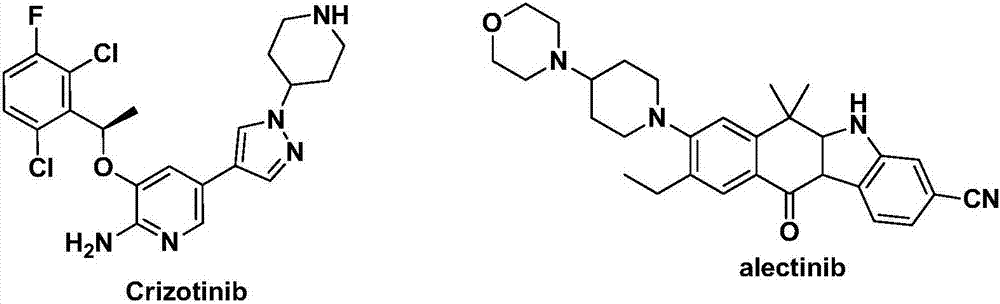
Heterocyclyl-substituted indoxaphthalenone derivatives and their medical use
A technology of uses and organisms, applied in the fields of drug combination, anti-tumor drugs, organic chemistry, etc., can solve the problems of Crizotinib bioavailability to be improved, Crizotinib resistance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0038] Preparation of Example 1 Compound S1
[0039]
[0040] For the synthesis of compound 1-1, refer to CN102459172.
[0041] The synthesis of compound 1-2 refers to J.Med.Chem.2011, 54, 6342-6363.
[0042] Synthesis of Compound S1:
[0043] Dissolve compound 1-1 and 1.3eq compound 1-2 in dry 1,4-dioxane, add 0.2eq tetraphenylphosphine palladium and a few drops of 2M sodium carbonate solution under nitrogen protection, and heat at 120°C in microwave 1h, after the reaction was complete, the insoluble matter was filtered off, and the filtrate was directly mixed with silica gel and put on the column, and the chloroform [CHCl 3 ]: Methanol [MeOH]=100:1~30:1 (volume ratio) to obtain compound S1.
[0044] 1 H NMR (300MHz, CDCl 3 +MeOD)δ8.33(dd,J=8.2,0.7Hz,1H),8.23(d,J=8.2Hz,1H),7.80(d,J=2.6Hz,2H),7.68(s,1H), 7.63(s,1H),7.47(dd,J=8.2,1.6Hz,1H),7.40(dd,J=8.2,1.4Hz,1H),4.32(m,1H),4.03(d,J=11.5Hz ,2H), 3.55–3.41(m,2H), 2.10–1.98(m,4H), 1.73(s,6H).
preparation Embodiment 2
[0045] Preparation of Example 2 Compound S2
[0046]
[0047] For the synthesis of compound 2-1, refer to CN102459172.
[0048] Synthesis of Compound S1:
[0049] The synthesis of compound S2 was the same as that of compound S1 except that compound 1-1 was replaced by compound 2-1.
[0050] 1 H NMR (300MHz, CDCl 3 +MeOD)δ8.29(d,J=8.1Hz,1H),8.11(s,1H),7.64(s,1H),7.53(s,2H),7.39(s,1H),7.35(d,J =8.1Hz,1H),4.29(m,1H),3.99(d,J=11.2Hz,2H),3.46(d,J=10.2Hz,2H),2.66(d,J=7.3Hz,2H), 2.02(s,4H), 1.65(s,6H), 1.17–1.07(m,3H).
preparation Embodiment 3
[0051] Preparation of Example 3 Compound S3
[0052]
[0053] Synthetic references of compound 3-1 J. Med. Chem. 2011, 54, 6342–6363.
[0054] Synthesis of compound 3-2:
[0055] The synthesis of compound S3 was the same as that of compound S2 except that compound 3-1 was used instead of compound 1-2.
[0056] Synthesis of compound S3:
[0057] Dissolve compound 3-2 in dichloromethane, add trifluoroacetic acid, and stir at room temperature. After the reaction was complete, the reaction solution was distilled off under reduced pressure, then ethyl acetate and saturated sodium bicarbonate were added for extraction, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate and spin-dried to obtain compound S3.
[0058] 1 H NMR (300MHz, CDCl 3 +MeOD)δ8.42(d,J=10.2Hz,1H),8.24(s,1H),7.72(s,1H),7.61(d,J=6.8Hz,2H),7.54–7.43(m,2H ),4.27(s,1H),3.26(s,2H),2.84–2.68(m,4H),2.20(d,J=10.7Hz,2H),2.06–1.89(m,2H),1.75(s, 6H), 1.21 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com