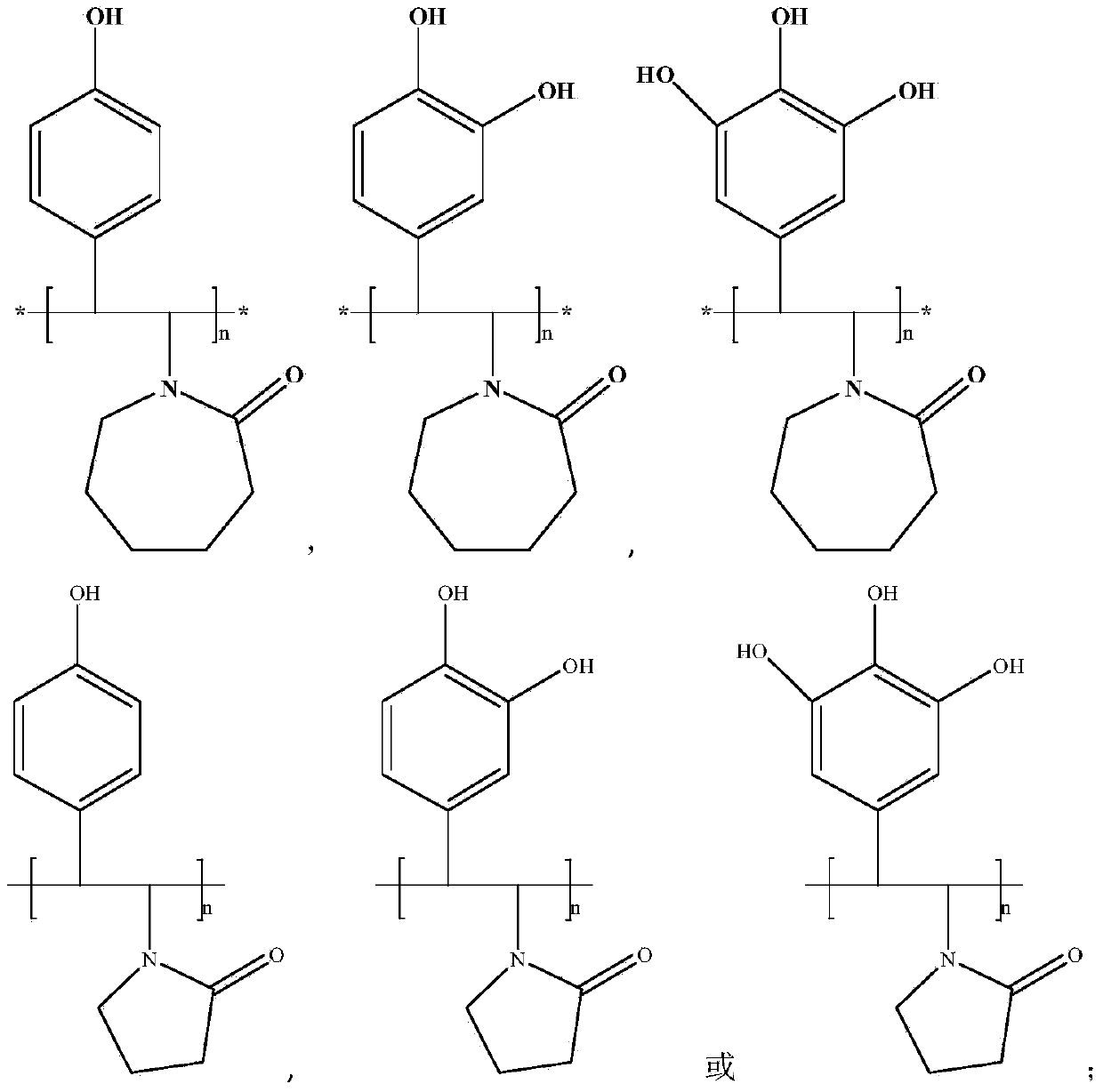
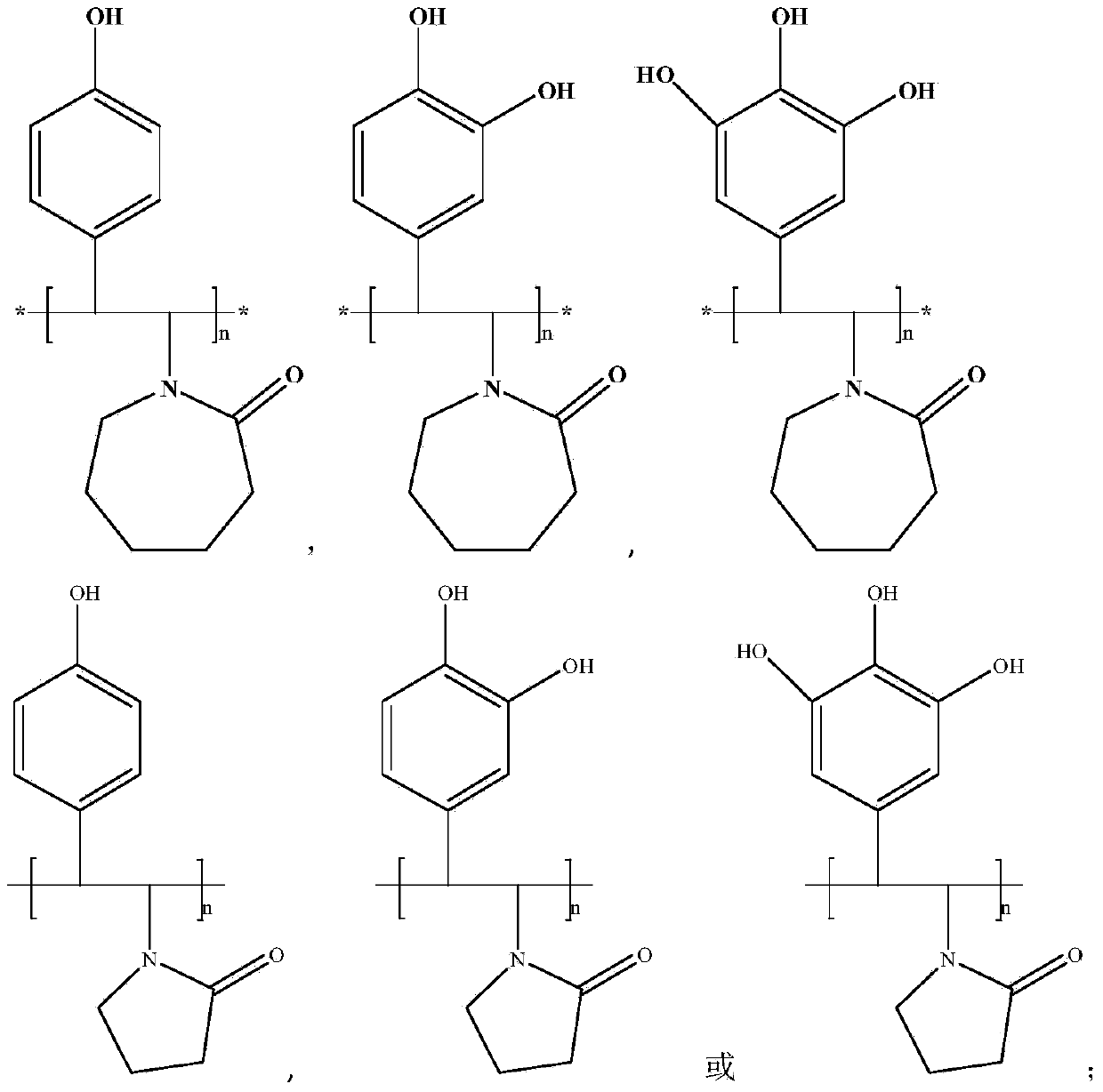
Novel hydrate kinetic inhibitor as well as preparation method and applications thereof
A kinetic inhibitor and hydrate technology, applied in chemical instruments and methods, drilling compositions, etc., can solve the problems of large loss of inhibitors, environmental damage, and large demand, so as to reduce the amount of production and prolong Time to build, good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Poly 1-(4-Hydroxystyrene) Caprolactam Homopolymer
[0027] Mix 20 grams of p-chlorophenol monomer and 0.3 grams of N-vinylcaprolactam monomer in a glass tube, use palladium as a catalyst, heat up to 60 ° C, keep the temperature for 4 hours, cool to room temperature, and then filter and distill the product to obtain 1-(hydroxystyryl) caprolactam monomer is obtained. Mix the obtained monomer 1-(hydroxystyryl)caprolactam and diethyl ether at a volume ratio of 1:1, and add azobisisobutyronitrile dropwise at 100°C with a concentration of 1.0wt% to initiate solution polymerization, and the reaction time is 6 hours , to obtain poly 1-(4-hydroxystyrene) caprolactam homopolymer.
[0028] The inhibitor described in the following examples was tested using the product obtained in Example 1, and the weight average molecular weight of the inhibitor was 300,000.
[0029] The experimental platform used in the following examples is described as follows:
[0030] The pr...
Embodiment 2
[0035] Based on the quality of water, add 150mL deionized water into the reactor, wait until the temperature in the reactor is stabilized at 1°C, and then inject gas (CH 4 92.05%, C 2 h 6 5.05%, C 3 h 8 2.90%), so that its pressure is 3MPa, the reaction reaches equilibrium, and the amount of methane gas consumed is reflected by the pressure drop. The results show that the induction time required for hydrate formation is 43 minutes, and the complete hydrate formation time is 151 minutes. Prepare 150mL of aqueous solution of poly 1-(4-hydroxystyrene) caprolactam (PHAO) with a mass concentration of 0.1% and put it into a reaction kettle. The temperature in the kettle is stabilized at about 1° C. 4 92.05%, C 2 h 6 5.05%, C 3 h 8 2.90%), so that the pressure was stabilized at 3MPa, and the reaction reached equilibrium. The results showed that the induction time required for hydrate formation was 139min, and the complete hydrate formation time was 380min.
Embodiment 3
[0037] Based on the quality of water, add 150mL deionized water into the reactor, wait until the temperature in the reactor is stabilized at 1°C, and then inject gas (CH 4 92.05%, C 2 h 6 5.05%, C 3 h 82.90%), so that its pressure is 9MPa, the reaction reaches equilibrium, and the amount of methane gas consumed is reflected by the pressure drop. The results show that the induction time required for hydrate formation is 35 minutes, and the complete hydrate formation time is 140 minutes. Prepare 150mL of aqueous solution of poly 1-(4-hydroxystyrene) caprolactam (PHAO) with a mass concentration of 0.3% and put it into a reaction kettle. The temperature in the kettle is stabilized at about 1° C. 4 92.05%, C 2 h 6 5.05%, C 3 h 8 2.90%), so that the pressure was stabilized at 9MPa, and the reaction reached equilibrium. The results showed that the induction time required for hydrate formation was 55 minutes, and the complete formation time of hydrate was 210 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com