Human serum CCL2 enzyme-linked immunosorbent assay kit as well as preparation and use methods thereof
An enzyme-linked immunosorbent assay and human serum technology, applied in the field of immunological detection, can solve the problems of insufficient sensitivity and accuracy for clinical application, unstable kit quality, unsuitable time-consuming operation, etc., and improve the level of clinical diagnosis and treatment. , low cost, short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
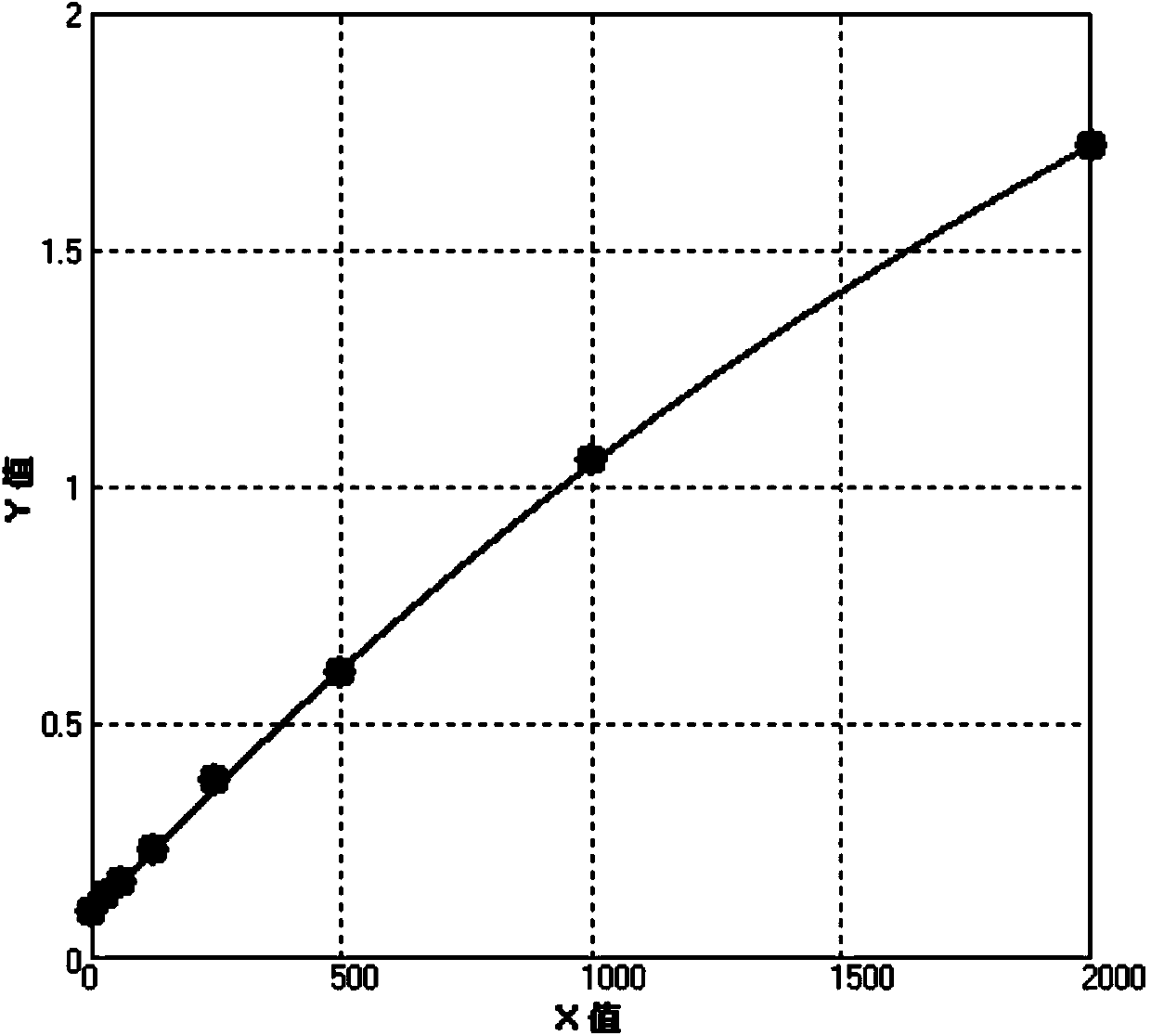
[0037] Preparation of the above-mentioned CCL2 standard: take recombinant human CCL2 / MCP-1 (279-MC-010) as a standard, take 25 μg of recombinant human CCL2 / MCP-1 (279-MC-010) and add 1 ml of 1% BSA Fully dissolve the PBS solution to obtain a standard solution with a liquid concentration of 10 μg / ml, dilute it 5000 times to 2000 pg / ml, take 2000 pg / ml as the highest point concentration, and then do 2-fold dilution, and dilute 6 gradients in total to obtain 1000pg / ml, 500pg / ml, 250pg / ml, 125pg / ml, 62.5pg / ml and 31.25pg / ml, in addition, take the dilution of the standard as the lowest concentration, ie 0pg / ml. The diluent of the standard here is phosphate buffer saline (PBS) containing 0.1% bovine serum albumin (BSA) at pH 7.2-7.4.
[0038] Preparation of the above-mentioned biotin-labeled anti-human CCL2 antibody: using biotinylated human CCL2 / MCP-1 antibody (BAF279) as the second antibody, the stock solution concentration is 50 μg / ml, and pH7.2-7.4 containing 0.1% bovine serum w...
Embodiment 1
[0063] (1) Some important conditions of the human serum CCL2 ELISA kit are as follows: coat with mouse anti-human CCL2 monoclonal antibody at a concentration of 2000ng / ml, and the concentration of biotin-labeled anti-human CCL2 antibody is 125ng / ml;
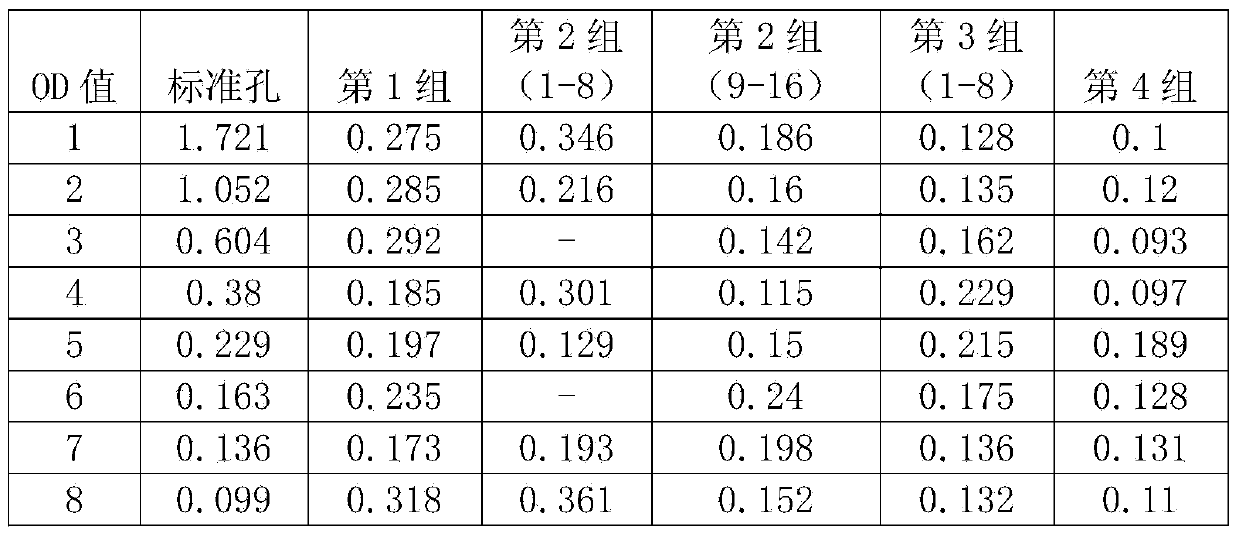
[0064] (2) Test sample introduction: Group 1 is 8 breast cancer samples for comparison, Group 2 is 16 samples positive for tumor metastasis, Group 3 is 16 samples negative for tumor metastasis, and Group 4 is physical examination of Huanghe Hospital Control 8 copies;
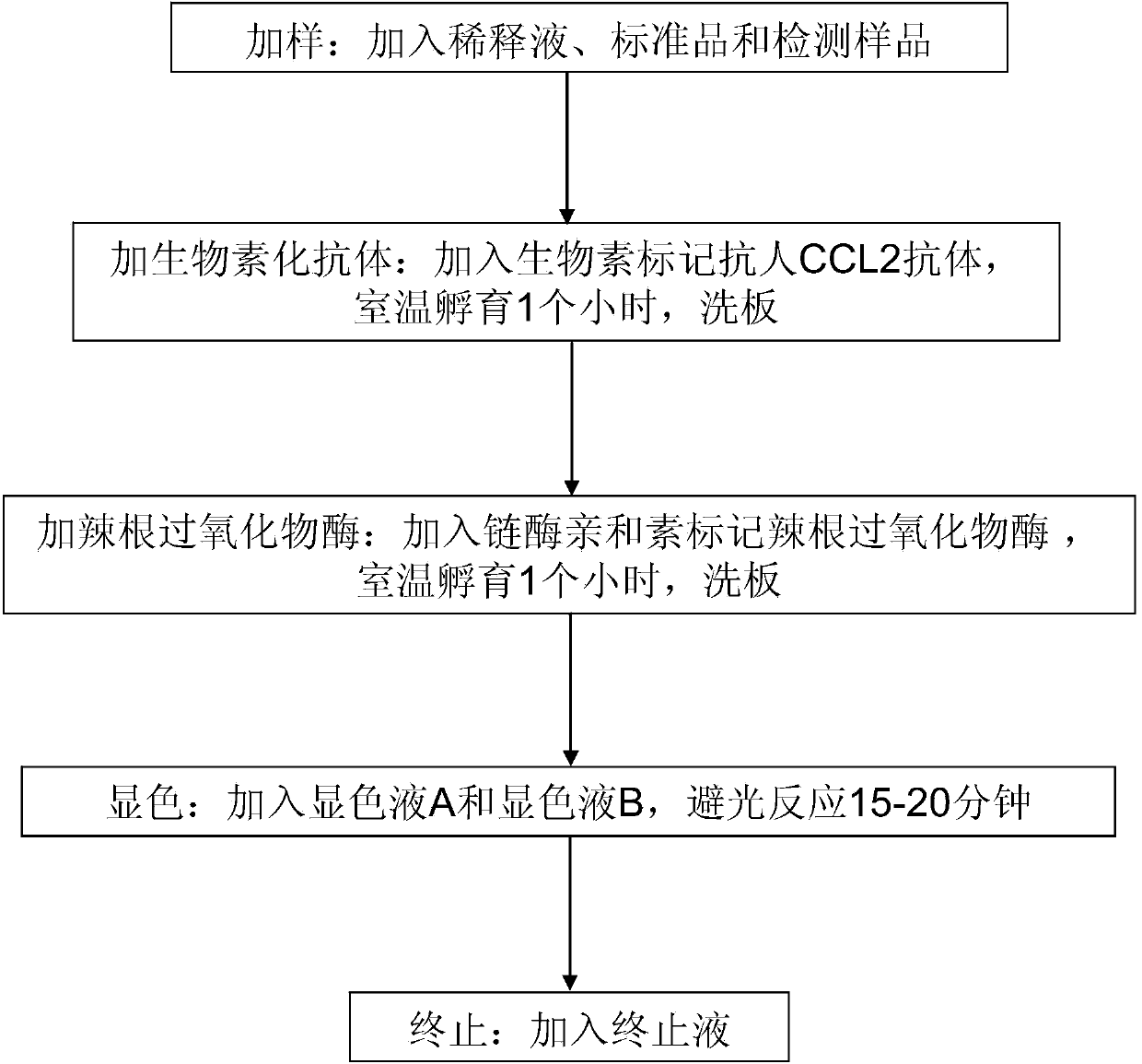
[0065] (3) Adding samples: Take the CCL2 standard product and add it to the enzyme-labeled reaction plate respectively, and add 100 μl to each well as a standard well; the enzyme-labeled reaction plate is a sample well except the above-mentioned standard well, and add 50 μl of diluent and 50μl test sample;
[0066] (4) Add biotinylated antibody: Add biotin-labeled anti-human CCL2 antibody to the standard well and sample well of the enzyme-labeled reaction plate, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com