Formulations of bendamustine
A bendamustine and antioxidant technology, applied in the field of bendamustine preparations, can solve the problems of stability differences of excipients, etc., and achieve the effect of overcoming stability differences and improving long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
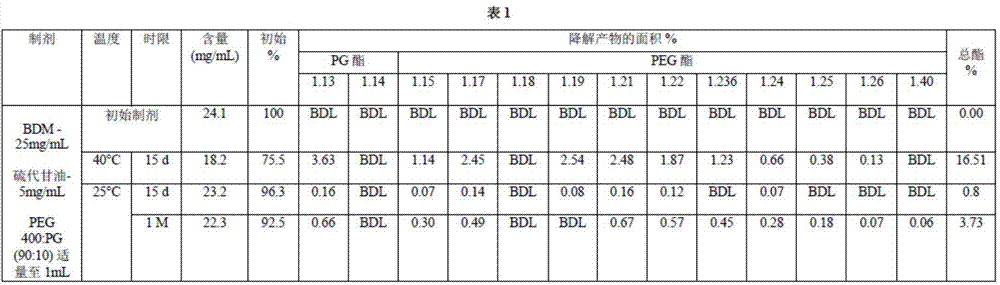
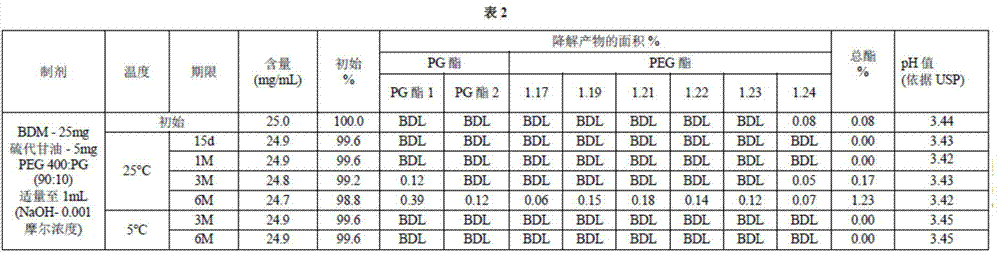
[0143]A mixture of PEG400 treated with sodium hydroxide was prepared by combining 200 μl of 1 N sodium hydroxide with PEG qs to 200 ml such that the NaOH concentration was 0.001 molar and mixing well. The pH of the PEG400 and NaOH mixture was determined according to the USP official monograph. A mixture of 5 g of PEG400 and sodium hydroxide was added to 100 ml of carbon dioxide-free water, and 0.3 ml of saturated potassium chloride solution was added. The pH was then measured to be 7.30, which is within the preferred range. A mixture of PEG:PG (90:10) was prepared by combining 20ml of a mixture of PG with PEG400 and sodium hydroxide qs to 200ml. Thioglycerol at a concentration of 5 mg / ml was added to 60 ml of a mixture of PEG:PG (90:10) and mixed well. Then bendamustine hydrochloride at a concentration of 25 mg / ml was added to 40 ml of a mixture of PEG:PG (90:10) and thioglycerol and mixed well. Additional PEG:PG (90:10) solution was added to bring the volume of the bendamu...
Embodiment 3
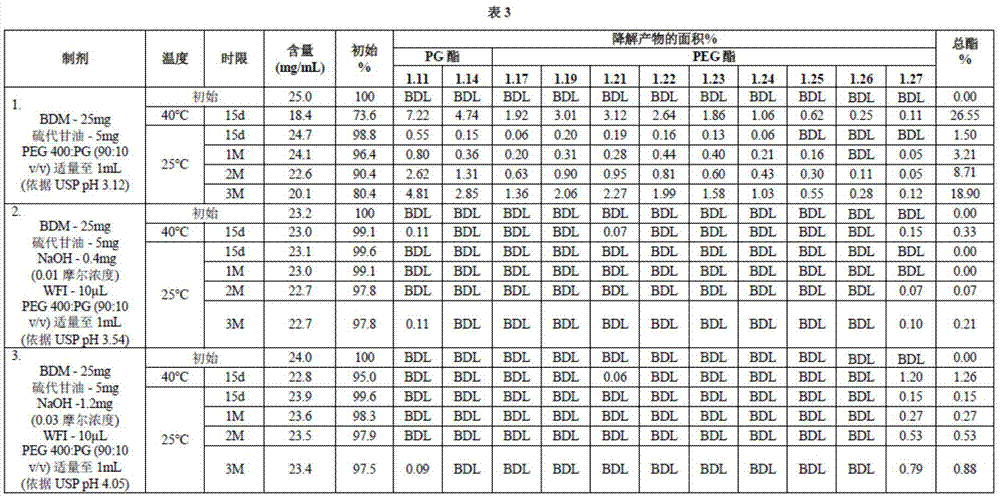
[0146] A mixture of PEG:PG (90:10) was prepared by combining 10ml of PG and PEG400 qs to 100ml. Add thioglycerol at a concentration of 5 mg / ml to 80 ml of PEG:PG (90:10) mixture and mix well. Then bendamustine hydrochloride at a concentration of 25 mg / ml was added to 40 ml of a mixture of PEG:PG (90:10) and thioglycerol and mixed well. Except for one sample where no sodium hydroxide was added (sample 1), the other two samples were prepared by adding 1 N sodium hydroxide solution to a PEG:PG (90:10) mixture to a concentration of 0.01 or 0.03 molar and mixing (for samples 2 and 3, respectively), such as image 3 (Table 3). The 0.01 and 0.03 molar samples were different from the samples in Examples 1 and 2 where the sodium hydroxide concentration was 0.001 molar. Additional PEG:PG (90:10) mixture was added to bring the volume of the bendamustine-containing solution to 50 ml. Formulations containing bendamustine were filtered and transferred to 5cc vials containing 4ml each. ...
Embodiment 4
[0150] A mixture of PEG400 with sodium hydroxide was prepared by combining 0.1 ml, 0.2 ml or 0.3 ml (samples 5, 6 and 7 respectively) of 1 N sodium hydroxide and PEG qs to 200 ml. The pH of the mixture of PEG400 and sodium hydroxide was determined according to the official monograph of USP. A mixture of 5 g of PEG400 and sodium hydroxide was added to 100 ml of carbon dioxide-free water, and 0.3 ml of saturated potassium chloride solution was added. The pH was then measured and the pH of the mixture of PEG400 and sodium hydroxide of sample 5 was 6.32. The pH of the mixture of PEG400 and sodium hydroxide for sample 6 was 7.30. The pH of the mixture of PEG400 and sodium hydroxide for sample 7 was 7.89. The pH of each PEG400 and NaOH mixture of Samples 5, 6 and 7 was within the preferred range. A mixture of PEG:PG (90:10) was prepared by combining 20 ml of PG with PEG400 qs to 200 ml, without sodium hydroxide (sample 4) or with sodium hydroxide at a concentration of 0.0005, 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com