Vaccine composition, and preparation method and application thereof
A technology of vaccine composition and bronchi, applied in the field of vaccine composition, can solve the problems of no obvious clinical symptoms, no detection of Bordetella bronchiseptica pathogen, etc., to increase the scope of application, good immune effect, reduce The effect of the stress response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
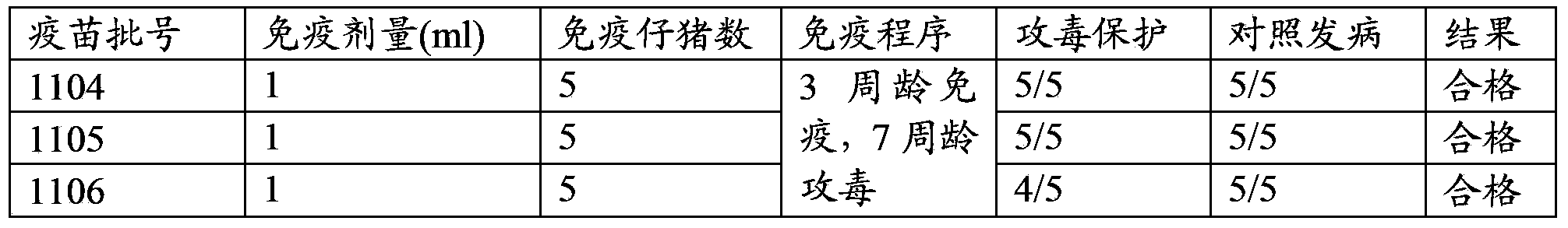
[0043] The preparation of the vaccine composition of embodiment 1 treatment and prevention mixed infection porcine atrophic rhinitis
[0044] 1. Preparation of bacterial solution
[0045] Streak the Bordetella bronchiseptica HN8 strain on the modified Bauer Jiang's blood plate, culture at 37°C for 48 hours, pick the single clone with obvious hemolytic ring in a test tube containing 5ml BHI, and shake at 150-220rpm, 37°C After 16-24 hours, the bacterial solution was expanded and cultivated at 37°C for 12-16 hours with a 1% inoculation amount, and the rotation speed was 150-220rpm. Samples were taken for a series of ten-fold dilutions, and counted on a plate.
[0046] Streak the strain of Pasteurella type D HB4 strain on the TSA plate, culture at 37°C for 16-20h, pick a single clone in a test tube containing 5ml of BHI, shake at 150-220rpm, 37°C for 16-24h, then The bacterial solution was expanded and cultivated at 37°C for 12-16 hours with a 1% inoculum size, and the rotation...
Embodiment 2
[0051] Example 2 Preparation of vaccine composition for treating and preventing mixed infection porcine atrophic rhinitis
[0052] The inactivation of bacterial classification and bacterial classification, the extraction of PMT toxoid and inactivation are the same as embodiment 1.
[0053] See Table 1 below for the ratio of each antigen in Example 2.
Embodiment 3
[0054] Example 3 Preparation of vaccine composition for treating and preventing mixed infection porcine atrophic rhinitis
[0055] The inactivation of bacterial classification and bacterial classification, the extraction of PMT toxoid and inactivation are the same as embodiment 1.
[0056] See Table 1 below for the ratio of each antigen in Example 3.
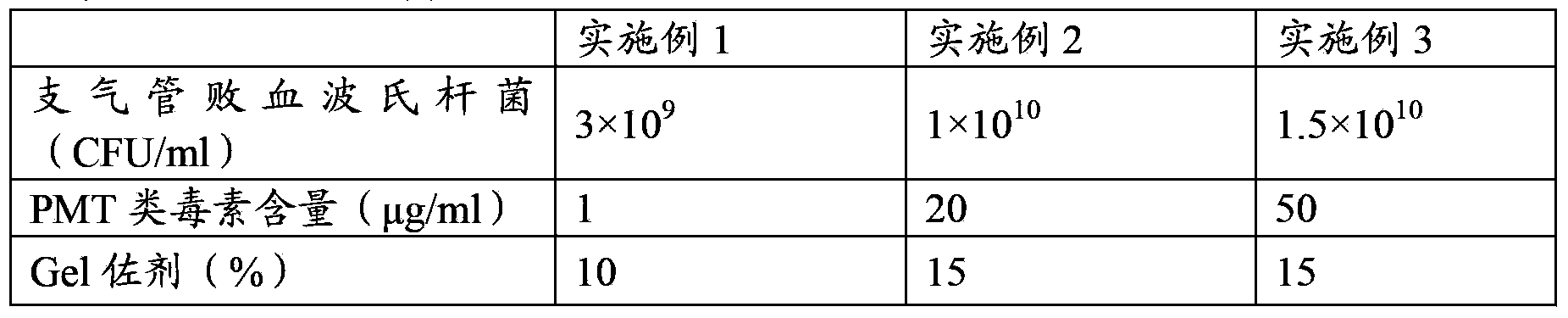
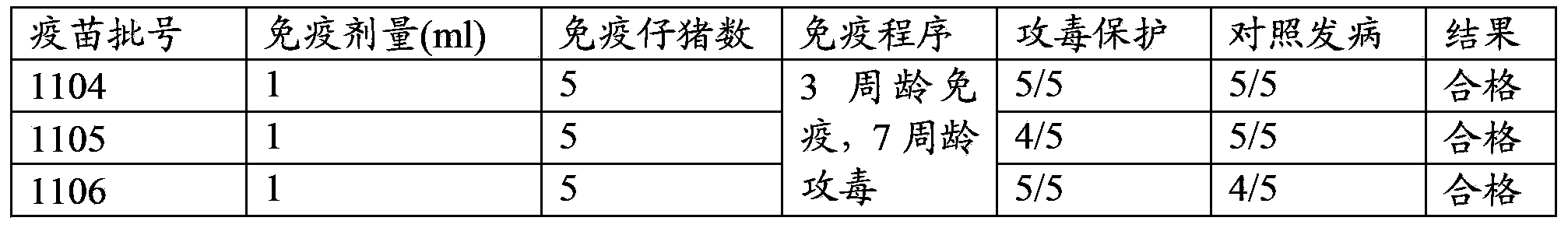
[0057] The concrete proportioning ratio of the vaccine of embodiment 1-3 is shown in Table 1:
[0058] The composition ratio of the vaccine composition of table 1 porcine atrophic rhinitis
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com