Aspirin‑arg‑gly‑asp‑val conjugate, its synthesis, nanostructure and application as drug delivery system
An arg-gly-asp-val, -arg-gly-asp-val technology, applied in the direction of non-active ingredients of polymer compounds, medical preparations containing active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
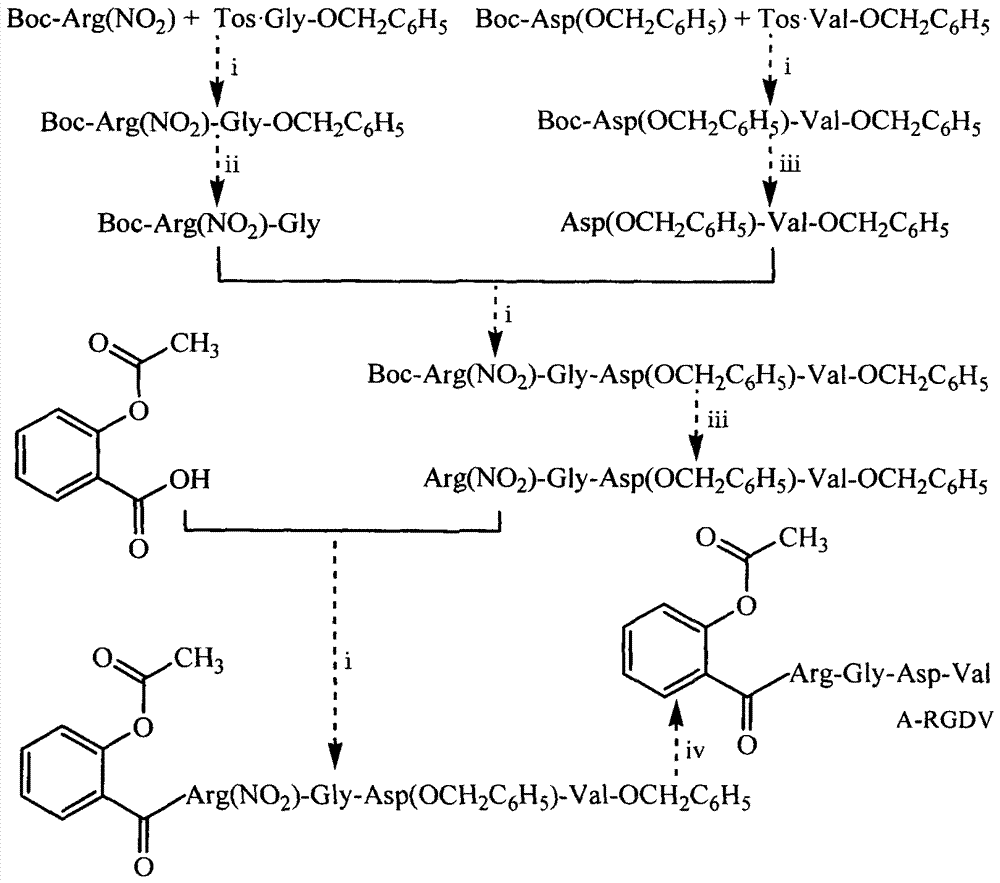
[0032] Embodiment 1 prepares Boc-Arg (NO 2 )-Gly-OBzl
[0033] 10.0g (31.3mmol) Boc-Arg (NO 2 ) was dissolved in 100ml of anhydrous tetrahydrofuran (THF), cooled in an ice bath, and 4.3g (31.3mmol) of N-hydroxybenzotriazole (HOBt) and 6.4g (31.3mmol) of dicyclohexylcarbonyldi imine (DCC), the reaction solution was stirred under ice bath for 10 min. Add 10.6g (31.3mmol) Tos·Gly-OBzl and 3.5g (31.3mmol) N-methylmorpholine (NMM), and stir at room temperature for 24h. The reaction mixture was filtered to remove dicyclohexylurea (DCU). The filtrate was concentrated under reduced pressure, and the residue was dissolved in 150 ml of ethyl acetate. The resulting solution was sequentially washed with 5% NaHCO 3 Washing with aqueous solution, washing with saturated NaCl aqueous solution, 5% KHSO 4 Washing with aqueous solution and saturated NaCl aqueous solution. Anhydrous Na for organic phase 2 SO 4 After drying and filtering, the filtrate was concentrated to dryness under red...
Embodiment 2
[0034] Embodiment 2 prepares Boc-Arg (NO 2 )-Gly
[0035] 11.85g (25.4mmol) Boc-Arg (NO 2 )-Gly-OBzl was dissolved in 50ml of methanol, cooled in an ice bath, 12ml of sodium hydroxide-methanol solution (1M) was added, stirred for 1 hour, the reaction solution was neutralized with 2M hydrochloric acid to pH=7, concentrated under reduced pressure to remove methanol, and the remaining The product was acidified with 2M hydrochloric acid to pH=2, and concentrated under reduced pressure to remove water. The residue was dissolved with 100 ml of ethyl acetate. The resulting solution was washed with saturated NaCl aqueous solution. Anhydrous Na for organic phase 2 SO 4 Dry, filter, and concentrate the filtrate to dryness under reduced pressure. The residue was triturated with a small amount of petroleum ether to obtain 8.9 g (93%) of the title compound, which was directly cast into the next reaction. ESI-MS(m / e): 377[M-H] - .
Embodiment 3
[0036] Example 3 Preparation of Boc-Asp(OBzl)-Val-OBzl
[0037]According to the method of Example 1, 10.7 g (89%) of the title compound was obtained as an oil from 7.7 g (23.7 mmol) of Boc-Asp (OBzl) and 9.0 g (23.7 mmol) of Tos.Val-OBzl. ESI-MS(m / e): 513[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com