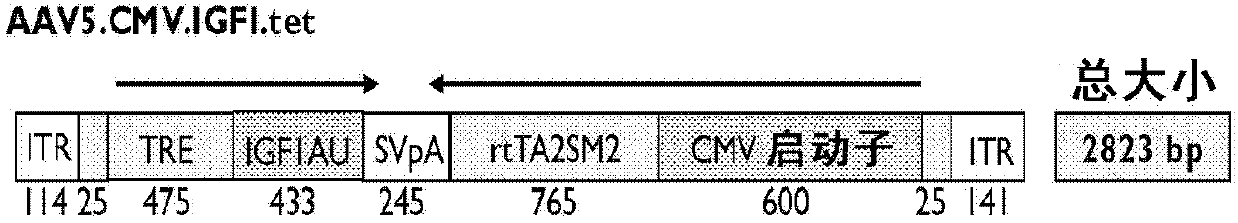
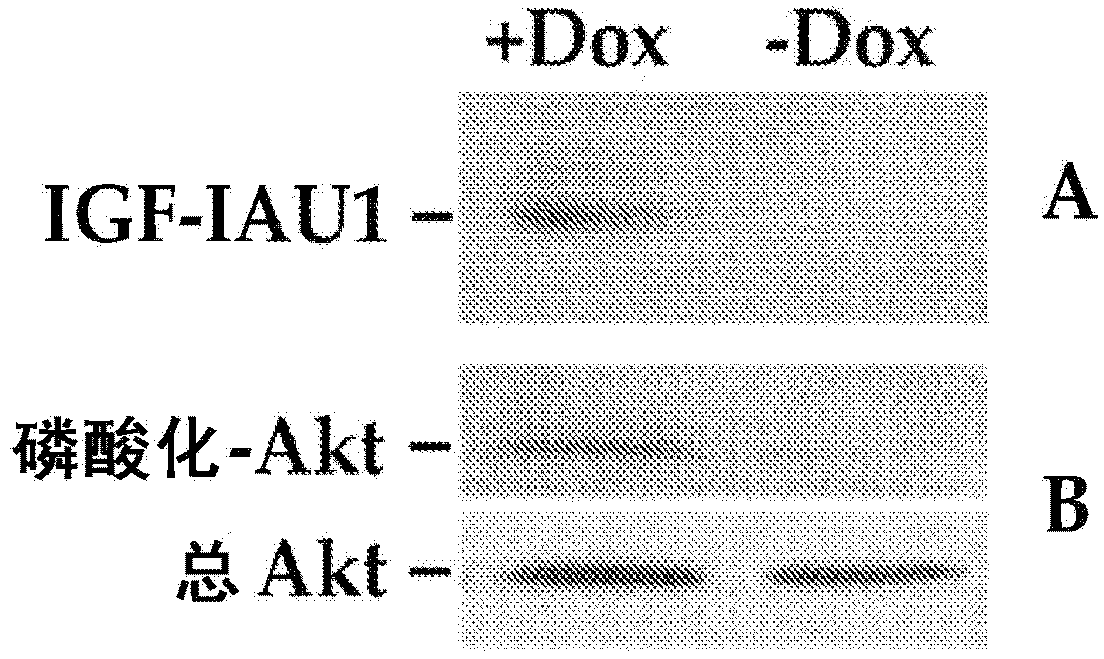
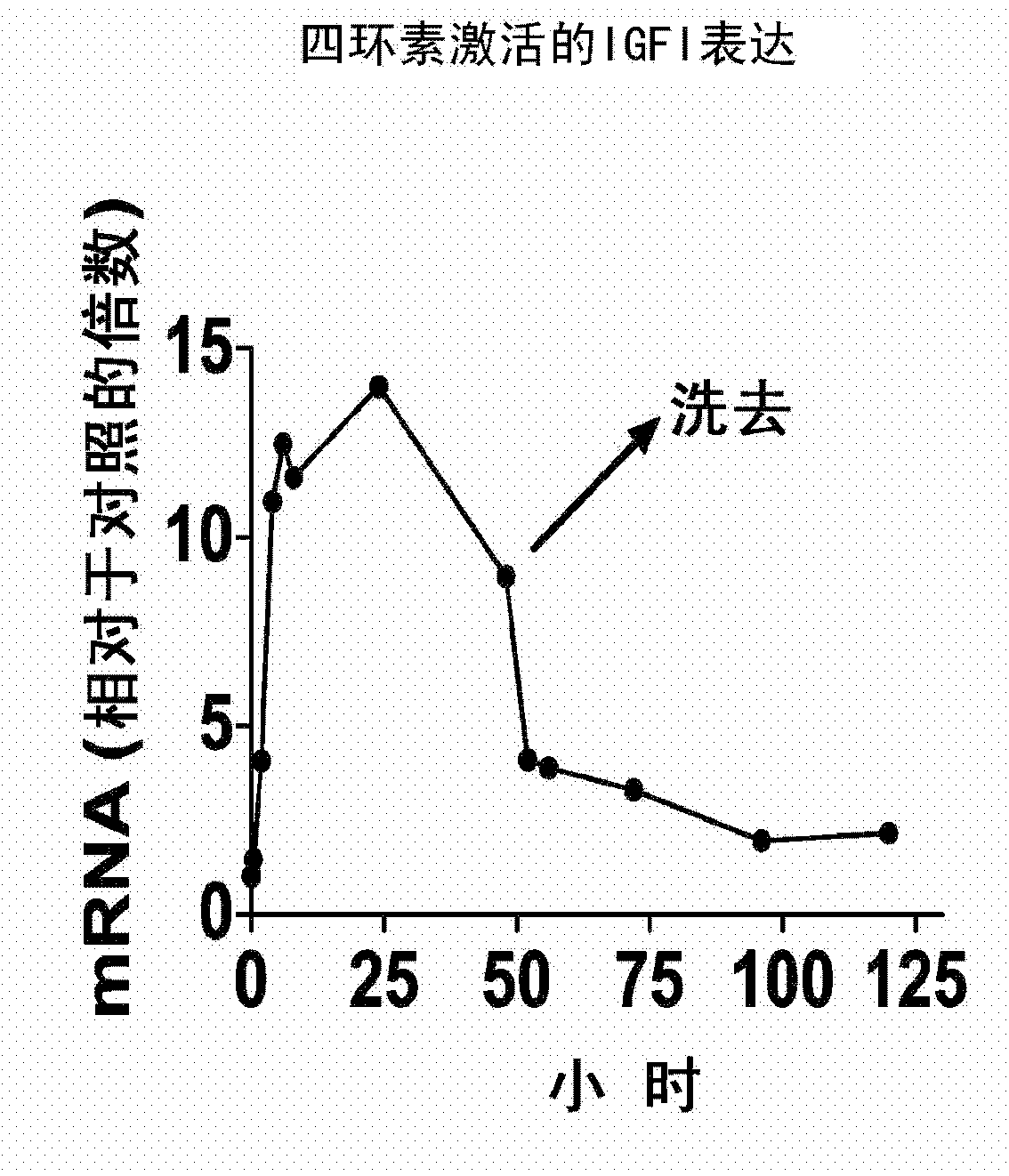
Systemic delivery and regulated expression of paracrine genes for cardiovascular diseases and other conditions
A technology of paracrine and paracrine polypeptides, which is applied in the fields of cell and molecular biology and medicine, and can solve the problems of shutting down transgene expression, not being able to treat heart disease, and not being able to regulate transgenes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Example 1: Intravenous delivery of AAV9 encoding urocortin-2 enhances cardiac function in normal mice
[0159] This example demonstrates the effectiveness of an exemplary embodiment of the invention: intravenous delivery of AAV9 / urocortin-2 (or AAV9 / UCn2) resulted in sustained increases in serum UCn2 and LV systolic function, demonstrating this exemplary embodiment of the invention. Effectiveness of a sexual embodiment in the treatment of heart failure.
[0160] In this study, we developed and tested the relative efficacy of two adeno-associated virus (AAV) serotypes (AAV5 and AAV9) encoding urocortin-2 (UCn-2), which -2 is a vasoactive peptide in the corticotropin-releasing factor family with diverse beneficial effects in animals and patients with heart failure. AAV5.Ucn-2 and AAV9.Ucn-2 (5×10 11 genome copies, gc). At four weeks (wk) after gene transfer, AAV DNA (qPCR) in liver (AAV5.UCn2: 2,601,839 copies / μg; AAV9.UCn2: 30,121,663 copies / μg) and heart (AAV5: 87,...
Embodiment 2
[0162] Example 2: Gene transfer for the treatment of cardiovascular disease
[0163] This example demonstrates the effectiveness of an exemplary embodiment of the invention in obtaining high yields of transgene expression in the heart in a manner that can be easily and safely administered.
[0164] In alternative embodiments, the invention provides methods of using expression vehicles (eg, vectors) encoding paracrine transgenes. In this embodiment, the transgene acts as a hormone and has a cardiac effect after release into the circulation from a distant site. In an alternative embodiment, this approach could circumvent the problem of achieving high yields of cardiac gene transfer and allow patients to be treated by systemic injection during office visits.
[0165] We tested multiple AAV serotype vectors and delivery methods and successfully completed a proof-of-concept study of paracrine gene transfer. Rats with severe dilated CHF received skeletal muscle delivery of an ad...
Embodiment 3
[0240] Example 3: Delivery of AAV8 Encoding Urocortin-2 Enhances Cardiac Function
[0241] This example demonstrates that in an alternative embodiment of the method of the invention, the paracrine transgene acts as a hormone and has a cardiac effect after being released into the circulation from a distant site. This exemplary approach circumvents the problem of achieving high yields of cardiac gene transfer and enables patients to be treated by systemic injection during office visits. Furthermore, this exemplary approach can eliminate the need for intravenous (IV) delivery of therapeutic peptides and thereby avoid repeated and prolonged hospitalizations, high morbidity, and substantial economic costs. In alternative embodiments, the most suitable vector to achieve these goals is adeno-associated virus type 8 (AAV8), which provides long-term and broad expression.
[0242] In an alternative embodiment of the method, urocortin-2, a recently discovered vasoactive peptide of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com