Production of muconic acid from genetically engineered microorganisms
A genetic modification and microbial technology, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, lyases, etc., can solve the problems of expensive, unusable, unstable multi-copy plasmids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
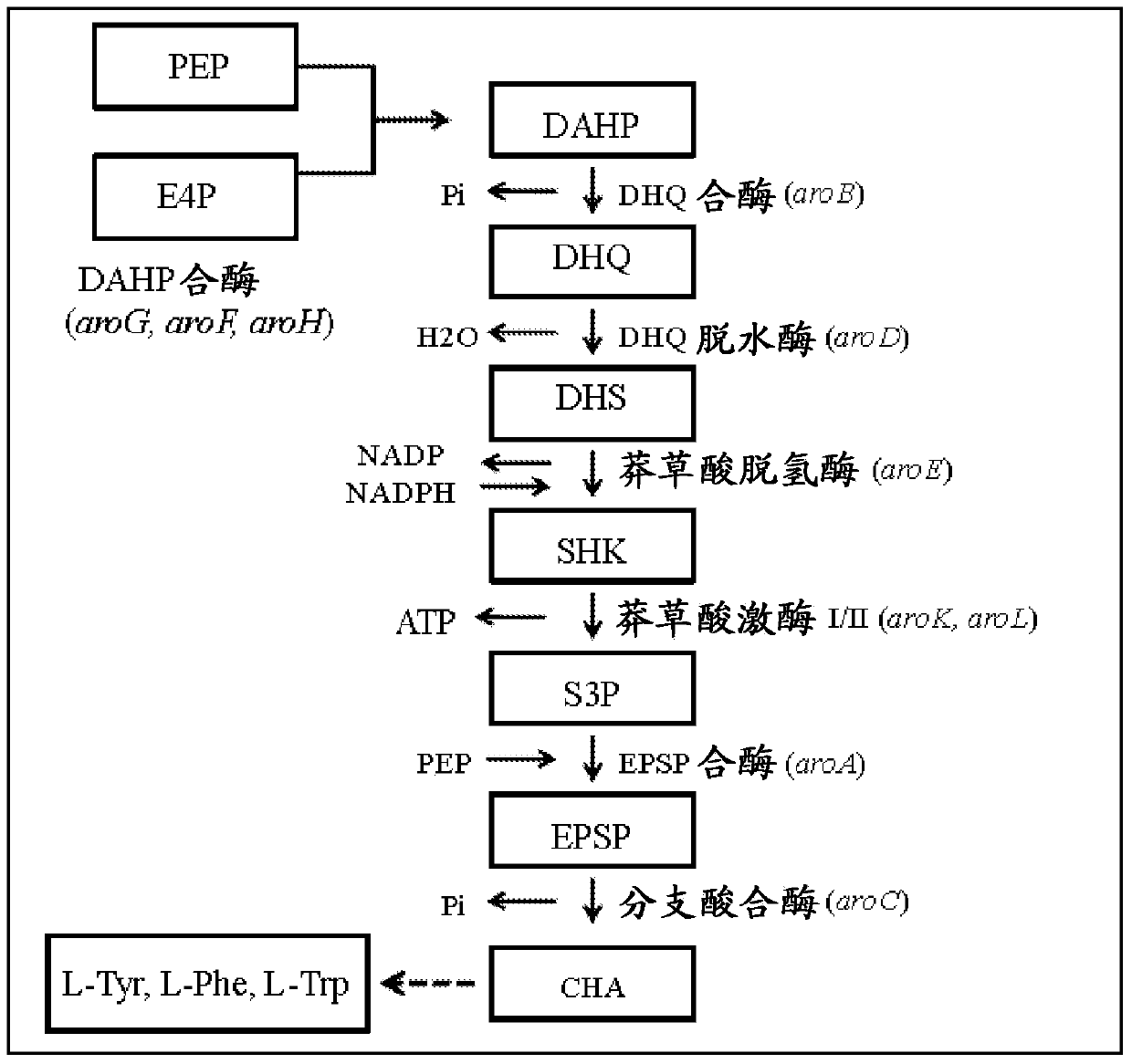
[0097] Increased expression of aroG and aroF
[0098] The tyrR gene of E. coli can be mutated by any of several well-known methods, such as chemical or radiation mutagenesis and selection (e.g., by PCR and DNA sequencing) or selection for analogue resistance (e.g., to 4-fluorotyramide Acid resistance), transposon mutagenesis, bacteriophage Mu mutagenesis or transformation. In preferred embodiments, the mutation in the tyrR gene is a null mutation (a mutation that results in no detectable activity), while in a more preferred embodiment, at least part of the tyrR gene is deleted. This can be achieved, for example, by utilizing a two-step transformation method using linear DNA molecules (Jantama et al., 2008a; Jantama et al., 2008b). In the first step, cam R The , sacB cassette is integrated at the tyrR locus to replace most or all of the tyrR open reading frame. In a second step, a linear DNA including a deletion of the tyrR gene was integrated by double panel selection for r...
Embodiment 2
[0101] Feedback-resistant AroG and AroF
[0102]Mutations in the aroG gene of the AroG enzyme (3-deoxy-D-arabinoheptonate-7-phosphate synthase or DAHPS) leading to feedback resistance are known in the art (Shumilin et al., 1999; Kikuchi et al. , 1997; Shumilin et al., 2002). Also known are methods to generate, identify and characterize these mutations (Ger et al., 1994, Hu et al., 2003). Preferred mutations are those that confer complete resistance to phenylalanine inhibition. Introduction of any of the known published feedback resistance mutations into the aroG gene contained in a chromosome or on a plasmid can be done by any of several known methods, an example of which is mutagenesis PCR, wherein the desired Mutations were synthesized as part of PCR priming oligonucleotides (Hu et al., 2003). Correct placement of mutations was confirmed by DNA sequencing. The sequence of the wild type aroG gene from E. coli C is given in SEQ ID No.18. A preferred mutation is a point mu...
Embodiment 3
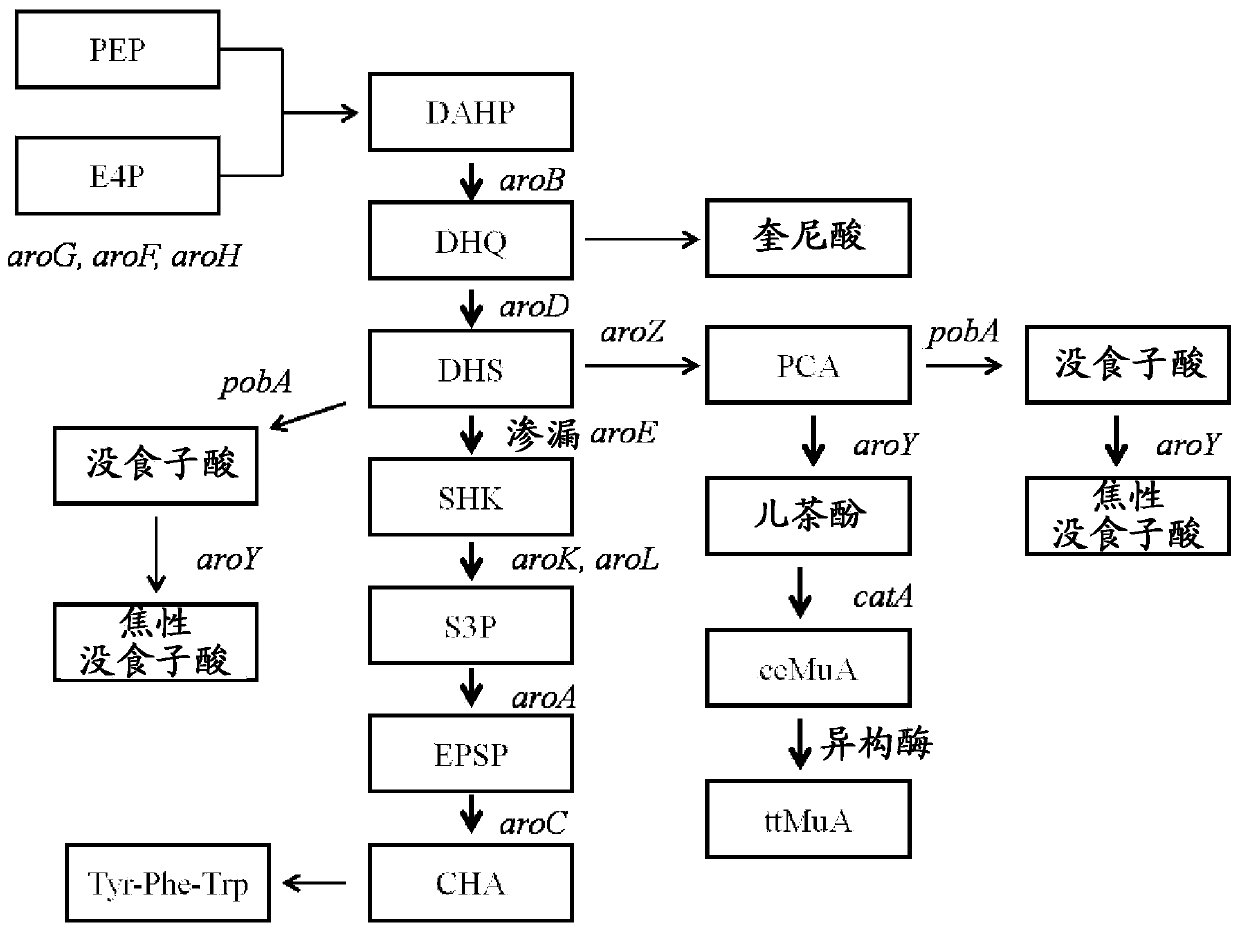
[0110] Deletion of aroE and muconate production from chromosomal DNA
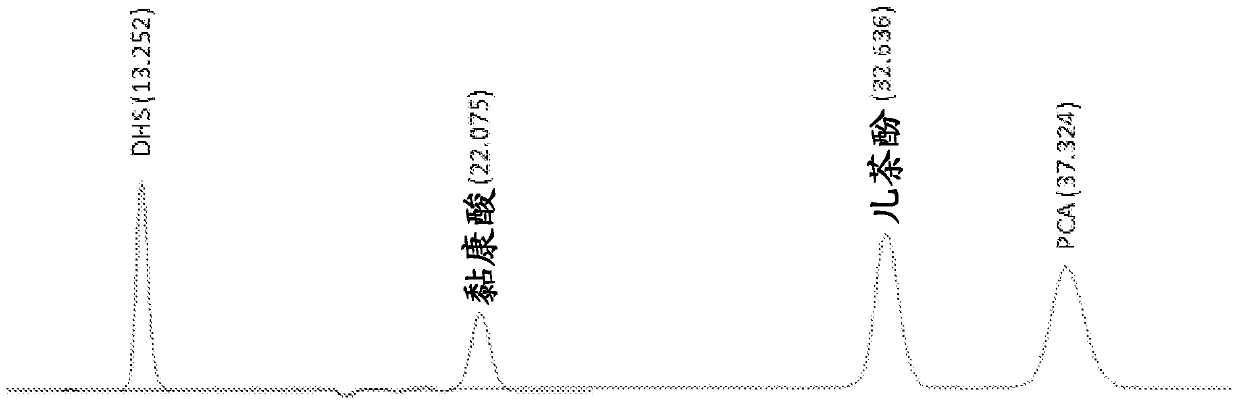
[0111]In this example the effect of overexpressing aroB and aroG on a multi-copy plasmid and expressing genes encoding proteins functional in the muconate pathway was investigated. Strain MYR34, containing a deletion in the aroE gene encoding shikimate dehydrogenase, was used as the parental strain in these studies. Deletion of the chromosomal copy of aroE was accomplished in a manner similar to that described in Example 1 above. When MYR34 was transformed with the plasmid pCP32AMP overexpressing the aroG gene encoding the DAHP synthase protein functional in the shikimate pathway, DHS accumulation was significantly enhanced. When MYR34 was transformed with a plasmid expressing the aroB gene from a constitutive promoter, no significant increase in DHS accumulation was noted. However, when E. coli strain MYR34 was transformed with a plasmid expressing both aroB and aroG genes, there was increased accumulati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com