Novel hydroxysafflor yellow A divalent medicinal salts, and preparing method and uses thereof
A technology of safflower yellow pigment and hydroxy safflower is applied in the field of hydroxy safflower yellow A bivalent medicinal salt and preparation thereof, freeze-dried powder injection and medicinal use, and the field of hydroxy safflower yellow medicinal salt, which can solve the problem of The product purity is not high, the quality is uncontrollable, and the structural properties cannot be qualitatively determined, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
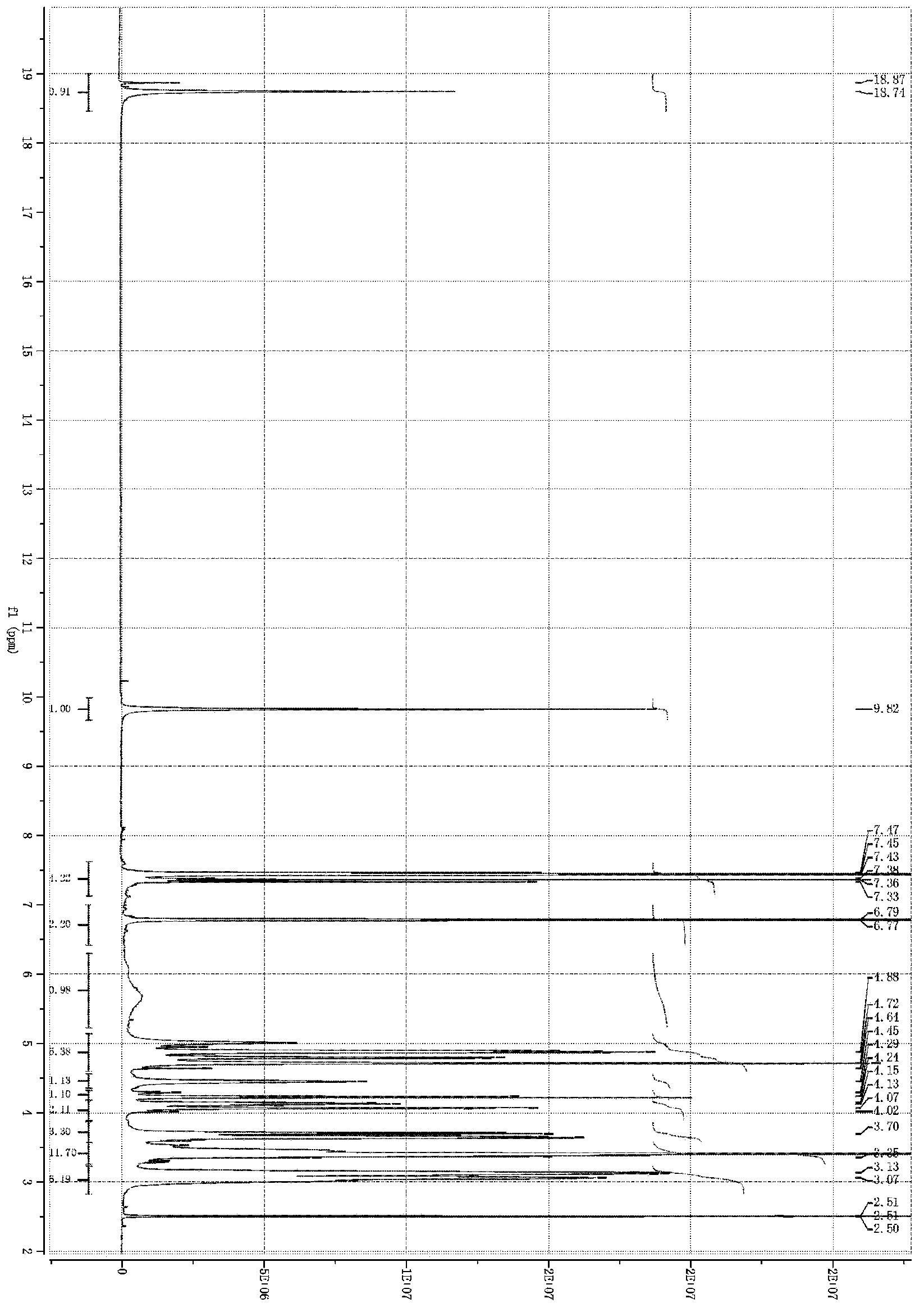
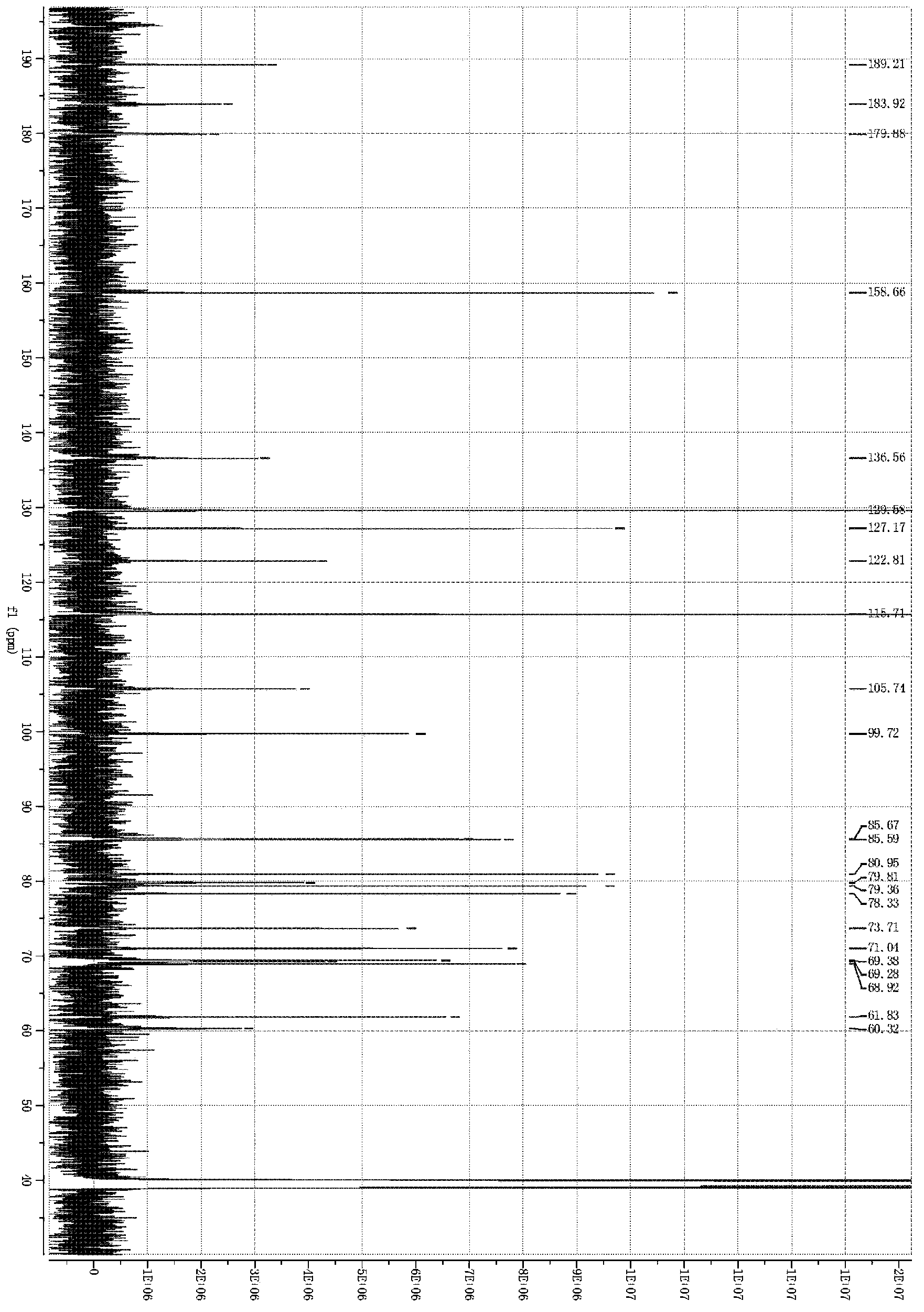
[0117] Embodiment 1: Hydroxysafflower yellow A calcium (in general formula I, R is calcium)
[0118] Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above centrifugate to the well-balanced 001*7 strong acid H-type cation exchange resin, the ratio of column diameter to height is 1:10, the column volume is 500ml, and the flow rate is 3ml / min. (OH) 2 Adjust the pH to about 5.5, and then slowly add to the macroporous adsorption resin separation column, the column diameter-to-height ratio is 1:12, and the sample loading flow rate is 10ml per minute. After loading the sample, it was eluted with deionize...
Embodiment 2
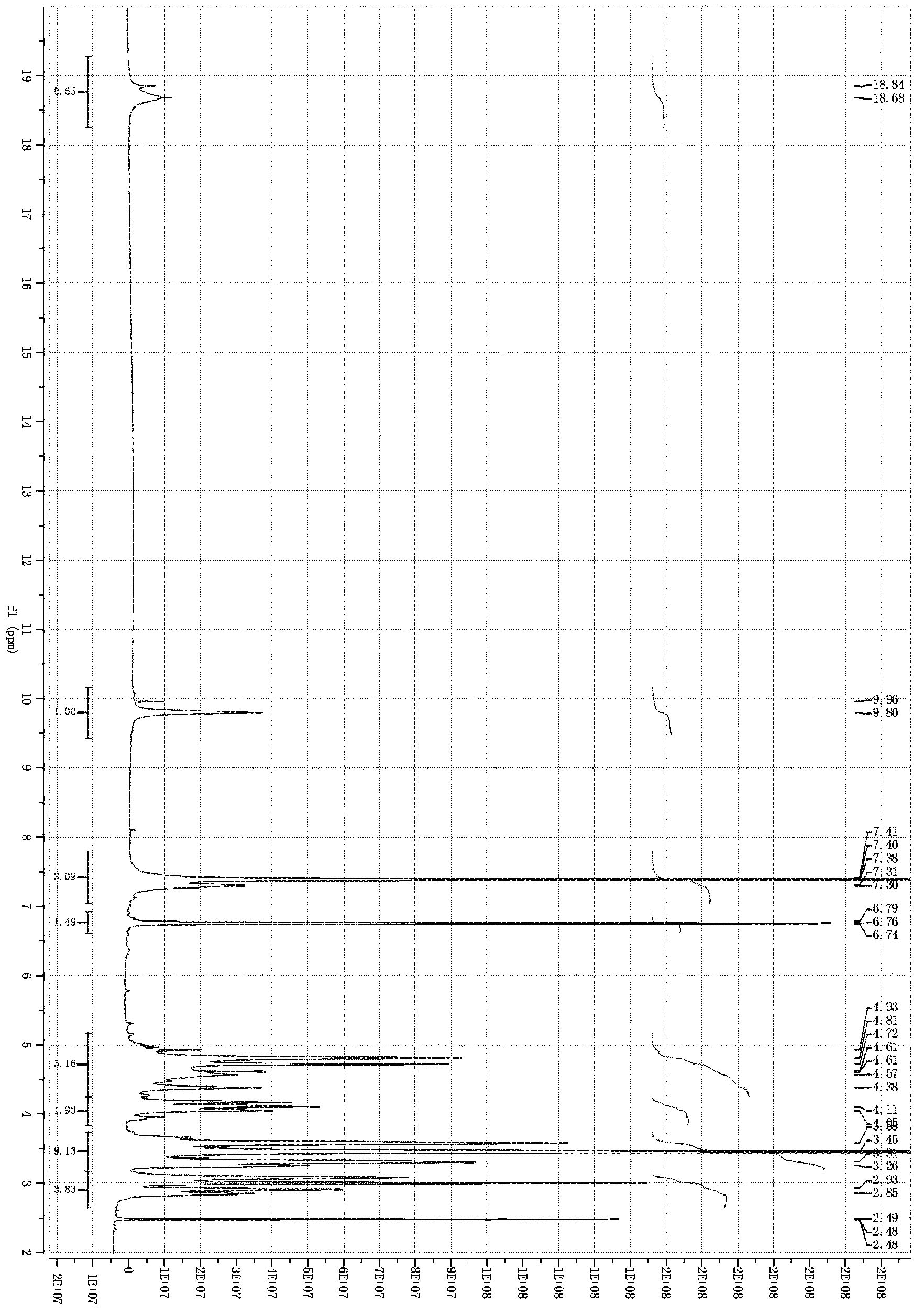
[0136] Embodiment 2: Hydroxysafflower yellow A calcium (in formula I, R is calcium)
[0137] Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above centrifugate to the HB-8 macroporous strongly acidic H-type cation exchange resin that has been treated and balanced. The column diameter-to-height ratio is 1:10, the column volume is 500ml, and the flow rate is 3ml / min. Use calcium hydroxide solution to adjust the pH to about 5.5, then slowly add to the macroporous adsorption resin separation column, the column diameter-to-height ratio is 1:12, and the sample loading flow rate is 10ml per minute. After ...
Embodiment 3
[0138] Example 3: Calcium Hydroxysafflor Yellow A
[0139] Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above-mentioned centrifugate into the macroporous adsorption resin separation column, the diameter-to-height ratio of the column is 1:12, and the sample loading flow rate is 10 ml per minute. After loading the sample, it was eluted with deionized water at room temperature at a flow rate of 20 ml per minute. The eluate was concentrated under reduced pressure at 60°C to obtain a concentrated solution of crude safflower yellow. In terms of safflower, 100ml of concentrated solution is obtained pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com