A kind of polypeptide and its preparation method and use
A technology of polypeptides and polypeptide fragments, applied in the field of polypeptides and their preparation, can solve the problems of low yield of AstressinB, high production cost of AstressinB, high market price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
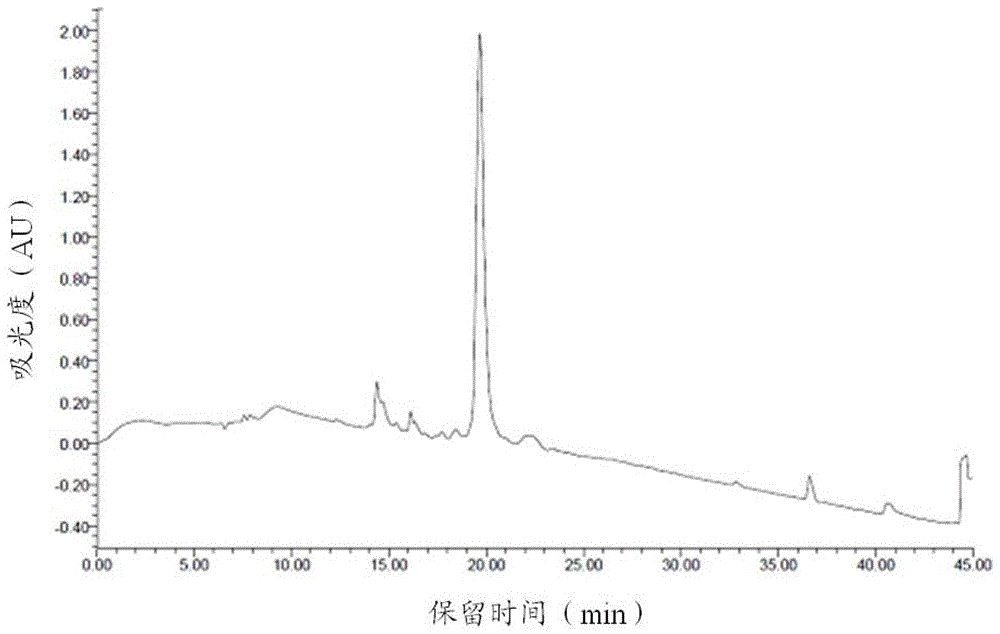
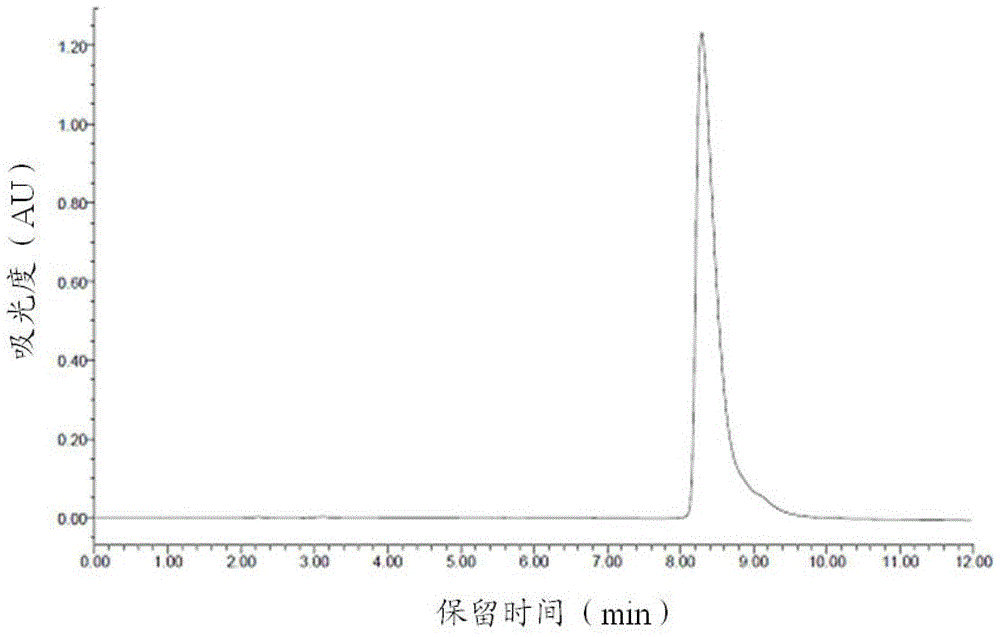
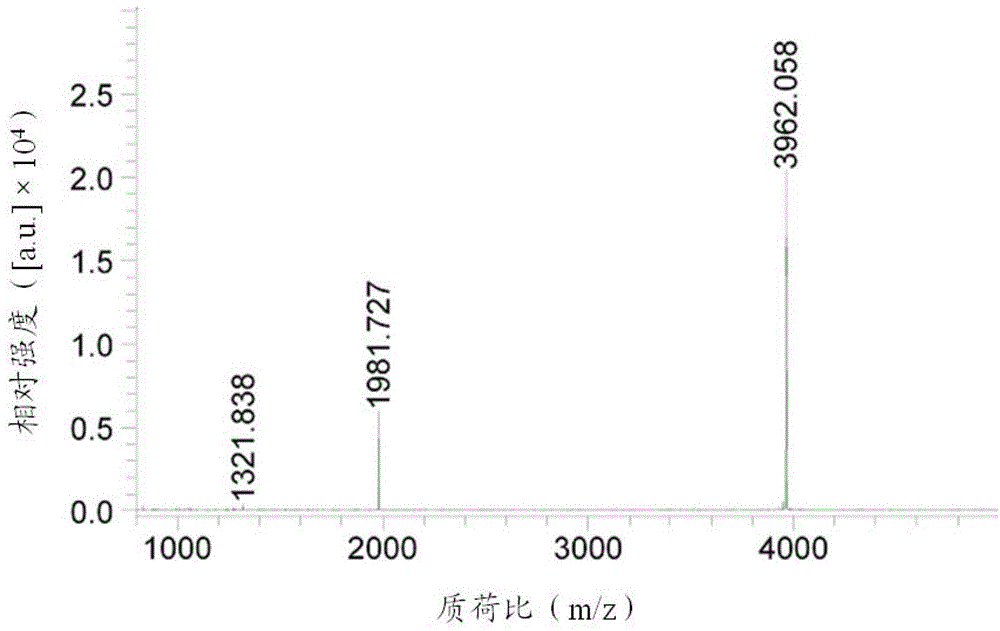
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of Polypeptide
[0082] Use the solid phase carrier of Rink Amide resin type, select Fmoc to protect the amino group strategy synthesis, and its operation steps are as follows:
[0083] Weigh 25 g (10 mmol) of Rink Amide resin, add it to a solid-phase reaction column, wash with DMF 2 to 3 times, and swell with DMF for 30 minutes. Use 100 mL of a mixture of piperidine and DMF with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc is completely removed. The color of the resin indicates that the Fmoc has been removed.
[0084] Weigh 10.6 g of Fmoc-Ile-OH, use DIEA as an activator, activate for 3 to 5 minutes, add to a reaction column and react for 1 to 3 hours to obtain Fmoc-Ile-Rink Amide resin.
[0085] Use 100 mL of a mixture of piperidine and DMF with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the F...
Embodiment 2
[0093] Example 2 Preparation of Polypeptide
[0094] Use the solid phase carrier of Rink Amide resin type, select Fmoc to protect the amino group strategy synthesis, and its operation steps are as follows:
[0095] Weigh 25 g of Rink Amide resin, add it to a solid-phase reaction column, wash it with DMF 2 to 3 times, and swell it with DMF for 30 minutes. Use 100 mL of a mixture of piperidine and DMF with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc is completely removed. The color of the resin indicates that the Fmoc has been removed.
[0096] Weigh 10.6 g of Fmoc-Ile-OH, use DIEA as an activator, activate for 3 to 5 minutes, add to a reaction column and react for 1 to 3 hours to obtain Fmoc-Ile-Rink Amide resin.
[0097] Use 100 mL of a mixture of piperidine and DMF with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc ...
Embodiment 3
[0105] Example 3 Preparation of Polypeptide
[0106] Use the solid phase carrier of Rink Amide resin type, select Fmoc to protect the amino group strategy synthesis, and its operation steps are as follows:
[0107] Weigh 25 g of Rink Amide resin, add it to a solid-phase reaction column, wash it with DMF 2 to 3 times, and swell it with DMF for 30 minutes. Use 100 mL of piperidine and DMF mixture with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc is completely removed. The resin has a color, indicating that the Fmoc has been removed.
[0108] Weigh 10.6 g of Fmoc-Ile-OH, use DIEA as an activator, activate for 3 to 5 minutes, add to a reaction column and react for 1 to 3 hours to obtain Fmoc-Ile-Rink Amide resin.
[0109]Use 100 mL of a mixture of piperidine and DMF with a volume ratio of 1:4 to remove the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com