Method for preparing graphene oxide through electrocatalytic oxidation
An electrocatalytic oxidation and fossil technology, applied in the electrolysis process, electrolysis components, etc., can solve the problems of output restriction and large-scale application, and achieve the effect of shortening the production cycle, low cost and small structural damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Graphene oxide was obtained by electrolyzing spectroscopically pure graphite rods in an electrolyte solution containing 0.9 mol / L sodium chloride and 0.65 mol / L sodium sulfate at a voltage of 13 V and at a temperature of 29°C for 5 hours.
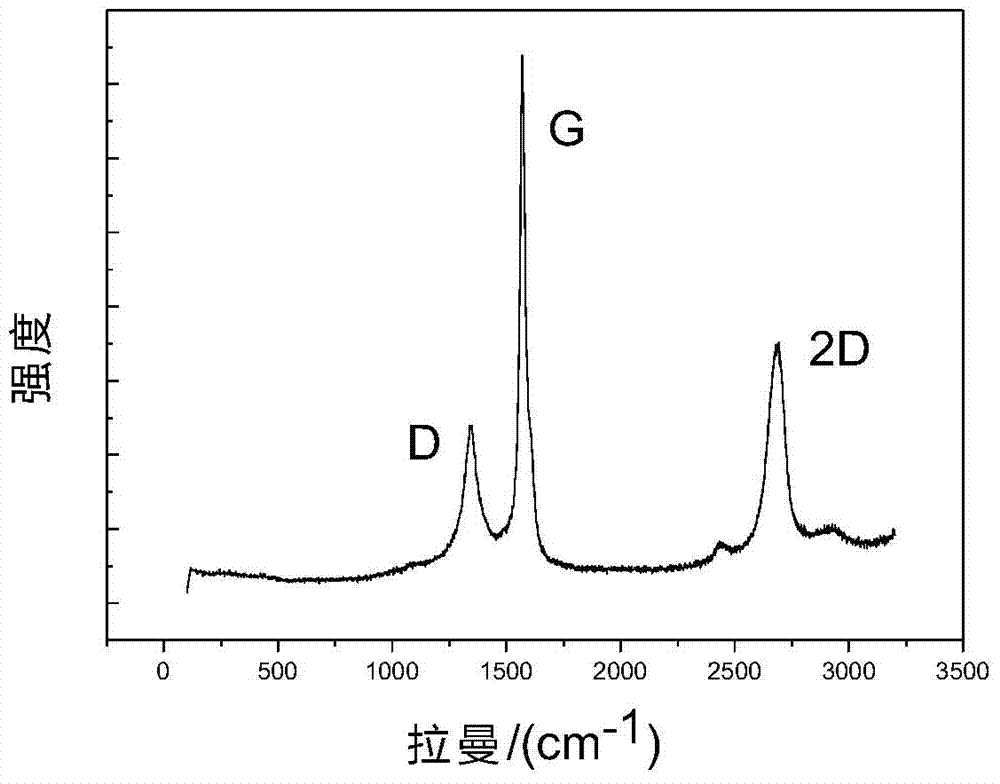
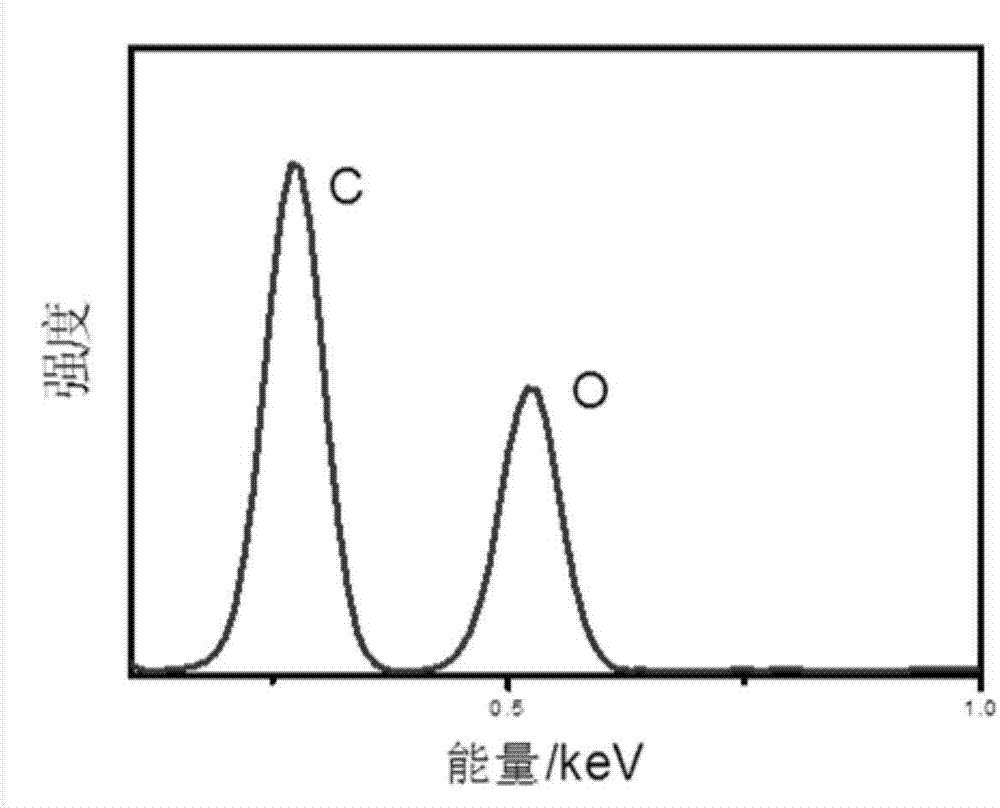
[0036] Adopt routine test method to test the graphene oxide that obtains, the result is as follows figure 2 and image 3 shown by figure 2 It can be seen from the Raman diagram that there are more obvious D peaks and 2D peaks in the exfoliated graphite oxide. The appearance of the 2D peak shows the existence of single-layer graphene in the product. In addition, the ratio of the D peak to the G peak is 0.33, which is much smaller than the ratio of the chemically synthesized graphite oxide (about 0.9). It proves that the structure of graphite oxide prepared by electrocatalysis is less damaged. Depend on image 3 The micro-area detection shows that the carbon-oxygen ratio of graphite oxide is about 3.8, which proves that graphite ...
Embodiment 2
[0039]Graphene oxide was obtained by electrolyzing spectroscopically pure graphite rods in an electrolyte solution containing 0.6 mol / L sodium chloride and 0.4 mol / L sodium sulfate, with a voltage of 20V and a temperature controlled at 35°C for 6 hours.
[0040] Adopt routine test method to test the graphene oxide that obtains, the result is as follows Figure 5 and Figure 6 shown by Figure 5 It can be seen from the Raman diagram in , that there are also obvious D peaks and 2D peaks in the exfoliated graphite oxide. The ratio of D peak to G peak is 0.52, which is also smaller than the ratio (about 0.9) of graphite oxide synthesized by chemical method. It also proves that the graphite oxide prepared by electrocatalysis has a small degree of structural damage. Depend on Figure 6 It can be seen from the micro-area detection that the carbon-to-oxygen ratio of graphite oxide can be obtained to be about 3.7, which proves that graphite has been oxidized to a certain extent.
...
Embodiment 3
[0043] Graphene oxide was obtained by electrolyzing spectroscopically pure graphite rods in an electrolyte solution containing 1.2 mol / L sodium chloride and 0.8 mol / L sodium sulfate at a voltage of 15 V and at a temperature of 25 °C for 4 hours.
[0044] Adopt routine test method to test the graphene oxide that obtains, the result is as follows Figure 8 shown by Figure 8 It can be seen from the Raman diagram in , that there are also obvious D peaks and 2D peaks in the exfoliated graphite oxide. The ratio of D peak to G peak is 0.14, which is much smaller than that of chemically synthesized graphite oxide (about 0.9). It proves that the structure of graphite oxide prepared by electrocatalysis is less damaged. Graphene oxide was characterized by infrared spectroscopy, the results are as follows Figure 9 As shown, obvious oxygen-containing functional groups appeared in the infrared spectrum, which have been marked in detail in the infrared spectrum.
[0045] Graphene oxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com