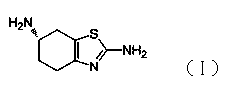
Preparation method of (6S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole
A technology of cyclohexanone and bromosuccinimide, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of endangering the health of operators, unfavorable industrial production, and high requirements for production equipment, so as to reduce waste and improve production Efficiency and shortening of the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of (6S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole
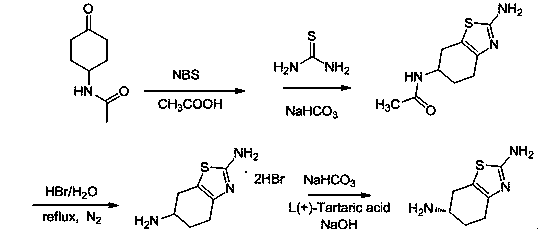
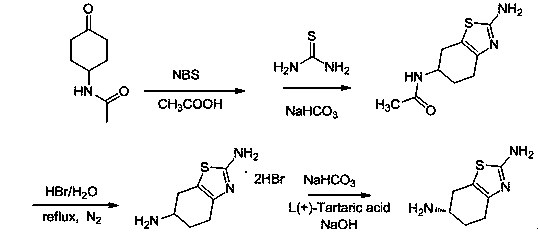
[0028] (1) Dissolve 9.3g (0.06mol) of acetamidocyclohexanone in 90ml of acetic acid and place it in a 150mL three-necked flask connected with a reflux device, heat up to 50°C under stirring conditions, and add N-bromosuccinyl 10.7g of amine, and keep the temperature at 50-60°C for 3 hours. The end point of the reaction is controlled by TLC. After the reaction of acetamidocyclohexanone is complete, add 9.12g (0.12mol) of thiourea, raise the temperature to 115°C, keep the temperature at 115~120°C for reflux reaction for 3 hours, and slowly cool down to room temperature under stirring. The solid was precipitated, filtered with suction, the filter cake was washed with water and methanol, and dried to obtain 9.2 g of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole (compound of formula B), yield 72.7% ;
[0029] (2) Put 9.2g (0.04354mol) of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole in ...
Embodiment 2
[0031] Example 2: Synthesis of (6S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole
[0032] (1) Dissolve 9.3g (0.06mol) of acetamidocyclohexanone in 90ml of acetic acid and place it in a 150mL three-necked flask connected with a reflux device, heat up to 50°C under stirring conditions, and add N-bromosuccinyl 10.7g of amine, and keep the temperature at 50-60°C for 3 hours. The end point of the reaction is controlled by TLC. After the reaction of acetamidocyclohexanone is complete, add 9.12g (0.12mol) of thiourea, raise the temperature to 115°C, keep the temperature at 115~120°C for reflux reaction for 3 hours, and slowly cool down to room temperature under stirring. The solid was precipitated, filtered with suction, the filter cake was washed with water and methanol, and dried to obtain 9.5 g of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole (compound of formula B), yield 75.1% ;
[0033] (2) Put 9.5g (0.050mol) of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole in a...
Embodiment 3
[0035] Embodiment three: (6S)-2, the synthesis of 6-diamino-4,5,6,7-tetrahydrobenzothiazole
[0036](1) Dissolve 9.3g (0.06mol) of acetamidocyclohexanone in 90ml of acetic acid and place it in a 150mL three-necked flask connected to a reflux device, heat up to 50°C under stirring, and add N-bromosuccinyl 10.7g of amine, and keep the temperature at 50-60°C for 3 hours. The end point of the reaction is controlled by TLC. After the reaction of acetamidocyclohexanone is complete, add 9.12g (0.12mol) of thiourea, raise the temperature to 115°C, keep the temperature at 115~120°C for reflux reaction for 3 hours, and slowly cool down to room temperature under stirring. The solid was precipitated, filtered with suction, the filter cake was washed with water and methanol, and dried to obtain 9.1 g of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole (compound of formula B), yield 87.0% ;
[0037] (2) Put 9.1g (0.0431mol) of 2-amino-6-acetamido-4,5,6,7-tetrahydrobenzothiazole in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com