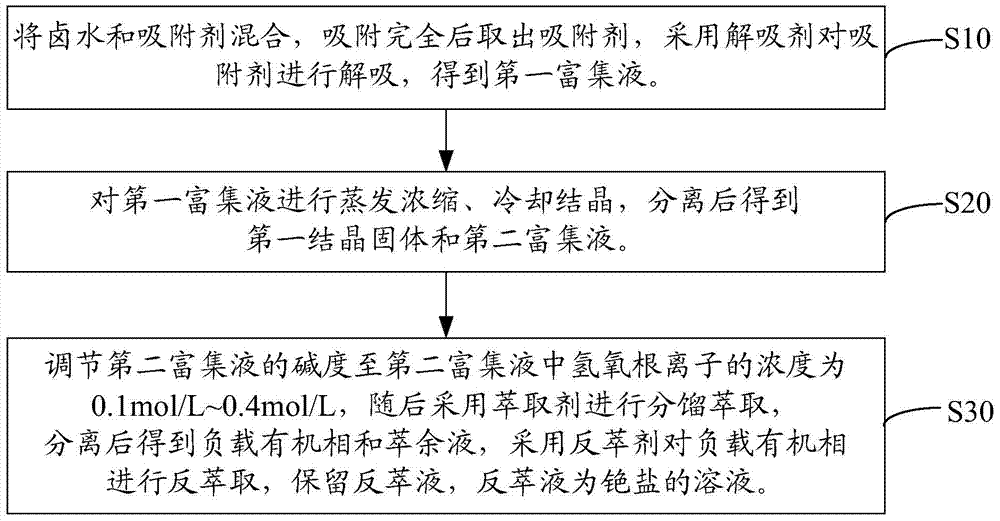
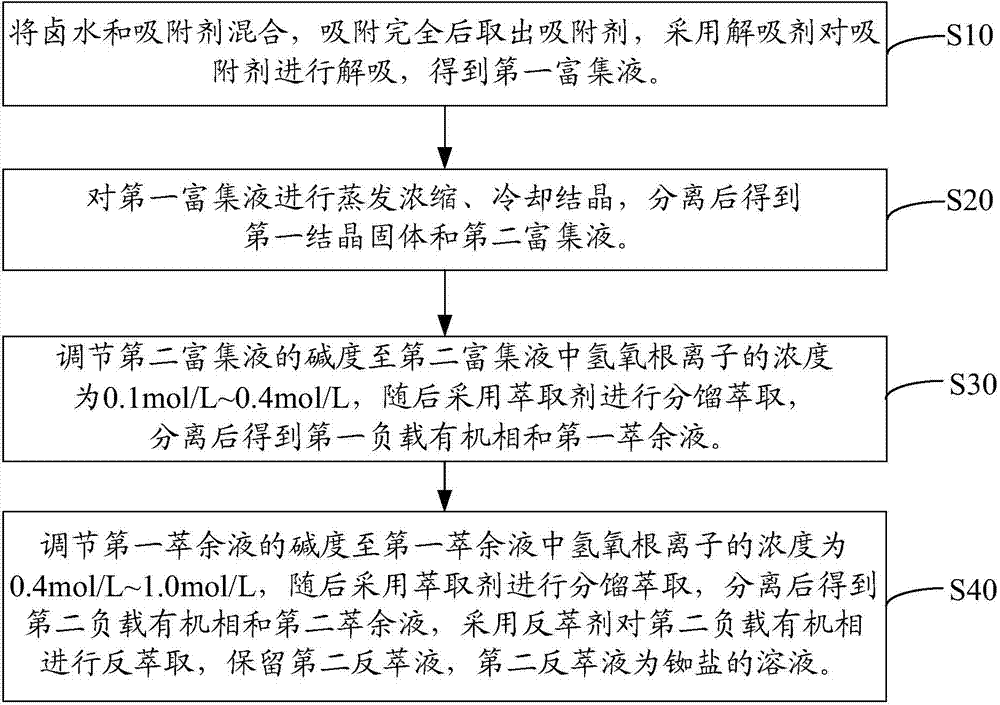
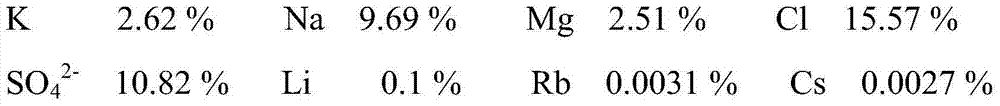Method for extracting rubidium salt from brine, and method for extracting cesium salt from brine
An extraction method and brine technology, which are applied in the extraction of cesium salts in brine, and the extraction of rubidium salts in brine, can solve the problems of inability to extract and separate rubidium salts and cesium salts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The source of brine in this example is sodium sulfate subtype salt lake brine, and the mass fractions of some elements in its composition are as follows:
[0066]
[0067] The extraction method of rubidium salt and cesium salt in brine is as follows:
[0068] Ammonium bicarbonate modified zeolite was loaded into the ion exchange column, the aspect ratio of the ion exchange column was 38, and the flow rate was 0.8mL / min. Brine is introduced until the ammonium bicarbonate modified zeolite reaches the saturated adsorption capacity, and the adsorption rates of potassium, rubidium and cesium are 90.21%, 93.42% and 92.96% respectively. Subsequently, the ammonium bicarbonate modified zeolite is desorbed by using ammonium bicarbonate as a desorbent to obtain a first enrichment solution containing potassium, rubidium and cesium. Among them, the resolution rates of potassium, rubidium and cesium are 98.84%, 99.01% and 99.33%, respectively.
[0069] The first enrichment solut...
Embodiment 2
[0075] The source of brine in this embodiment is chloride type deep underground brine, and the mass fractions of some elements in its composition are as follows:
[0076]
[0077] The extraction method of rubidium salt and cesium salt in brine is as follows:
[0078] Ammonium bicarbonate modified zeolite was loaded into the ion exchange column, the aspect ratio of the ion exchange column was 20, and the flow rate was 0.5mL / min. Brine is introduced until the ammonium bicarbonate modified zeolite reaches the saturated adsorption capacity, and the adsorption rates of potassium, rubidium and cesium are 91.27%, 94.38% and 95.66% respectively. Subsequently, the ammonium bicarbonate modified zeolite is desorbed by using ammonium bicarbonate as a desorbent to obtain a first enrichment solution containing potassium, rubidium and cesium. Among them, the resolution rates of potassium, rubidium and cesium are 98.24%, 98.89% and 99.45%, respectively.
[0079] The first enrichment solu...
Embodiment 3
[0085] The brine source in the present embodiment is oil brine, and the mass fraction of some elements in its composition is as follows:
[0086]
[0087] The extraction method of rubidium salt and cesium salt in brine is as follows:
[0088] Ammonium bicarbonate modified zeolite was loaded into the ion exchange column, the aspect ratio of the ion exchange column was 50, and the flow rate was 2.5mL / min. Pass brine until the ammonium bicarbonate modified zeolite reaches the saturated adsorption capacity, and the adsorption rates of potassium, rubidium and cesium are 89.56%, 92.08% and 93.34% respectively. Subsequently, the ammonium bicarbonate modified zeolite is desorbed by using ammonium bicarbonate as a desorbent to obtain a first enrichment solution containing potassium, rubidium and cesium. Among them, the resolution rates of potassium, rubidium and cesium are 98.78%, 99.01% and 99.20%, respectively.
[0089]The first enrichment solution was evaporated and concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com