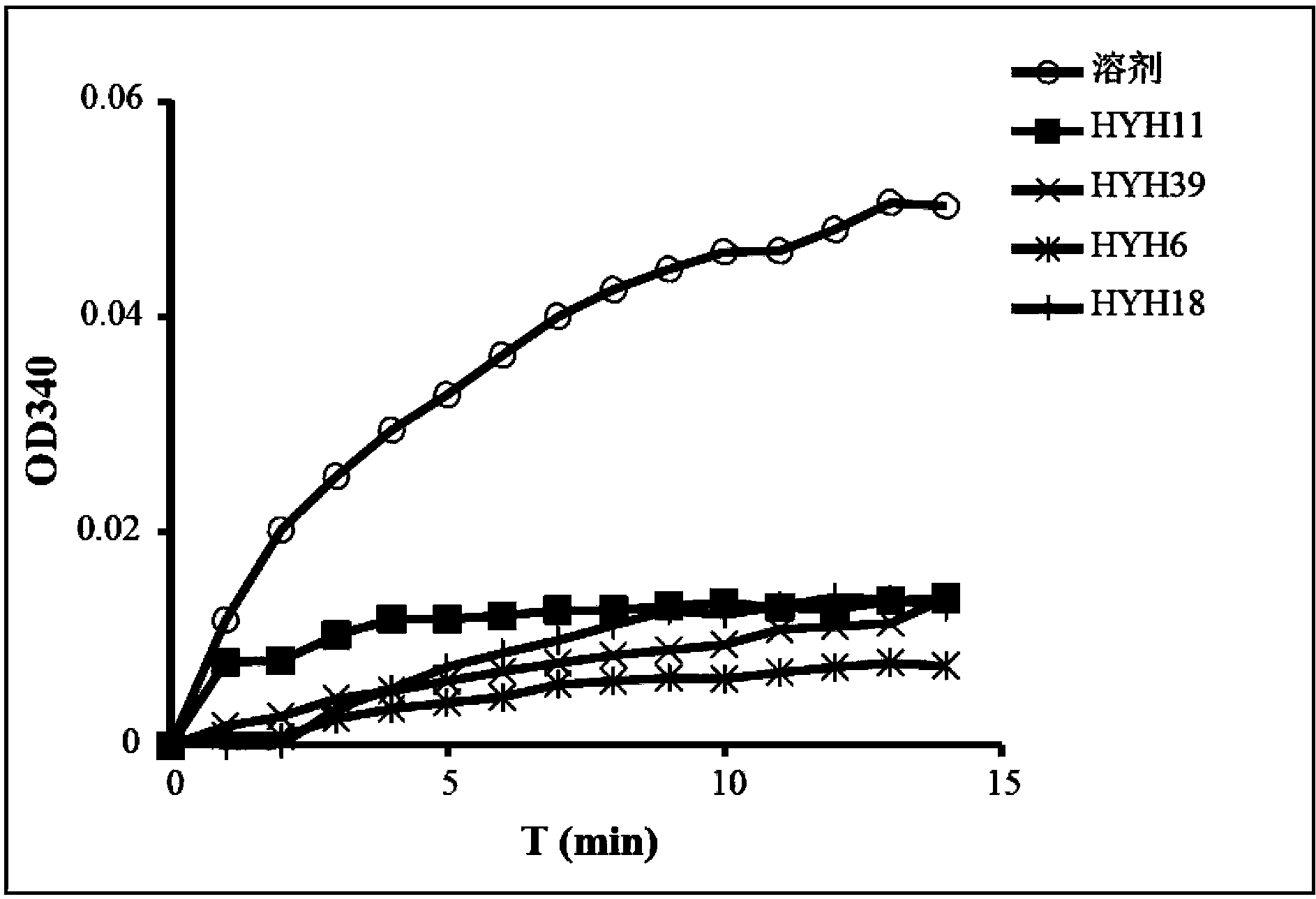
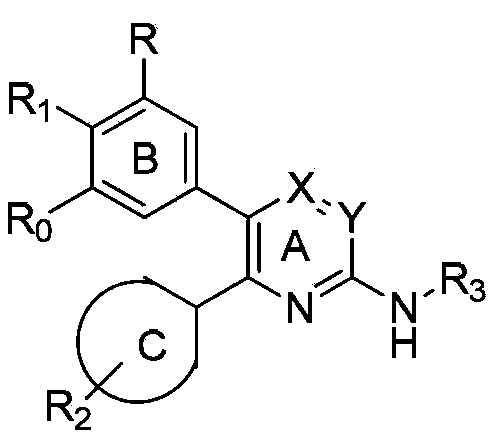
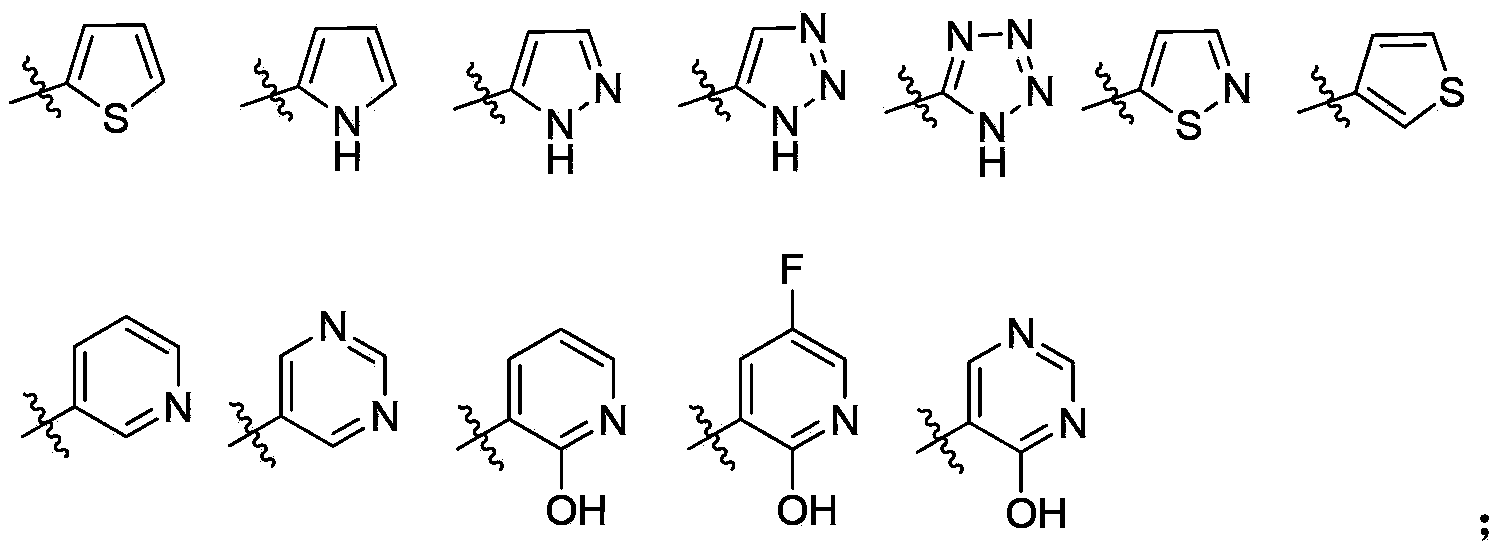
Aryl heterocycle micromolecule compounds, derivatives thereof, and preparing methods and uses of the compounds and the derivatives
A technology of small molecular compounds and compounds, applied in the field of medicine, can solve problems such as hindering development, narrow therapeutic window, and poor solubility of natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Preparation of compounds HYH11 and HYH57 (refer to the scheme shown in the following reaction formula)
[0109]
[0110] Step a: 2-Amino-6-chloropyridine (20g, 0.155mol, 1eq) was dissolved in DMF (300ml), at 0℃, N-bromosuccinimide (30.5g, 0.171mol, 1.1eq) Slowly add to the above mixture, react at room temperature for 2 hours, the reaction is complete. A large amount of water was added to precipitate a solid, filtered, washed with water, ethyl acetate / petroleum ether (V:V=1:4 (volume ratio)), and dried to obtain the crude product 2-amino-5-bromo-6-chloropyridine.
[0111] Step b: 2-Amino 5-bromo-6-chloropyridine (5.6g, 27mmol, 1eq) and N-ethylindole-5-boronic acid (5.36g, 28.3mmol, 1.05eq) were dissolved in toluene / ethanol (V :V=2:1) In the mixed solvent (75ml), add potassium carbonate (5.6g, 40.5mmol, 1.5eq) aqueous solution (56ml, 100mg potassium carbonate per ml of water) and protect with nitrogen, add tetrakistriphenylphosphonium palladium ( 1.6g, 1.35mmol,...
Embodiment 2
[0118] Example 2 Preparation of compound HYH1
[0119] It was prepared in the same manner as in Example 1, except that phenylboronic acid was substituted for 2-benzyloxy-5-fluorophenylboronic acid pinacol ester.
[0120]
[0121] 1 H NMR(300MHz, CDCl 3 )δ7.57(d,J=8.3Hz,1H),7.46(s,1H),7.36(d,J=3.0Hz,2H),7.21-7.13(m,4H),7.13-7.07(m,2H) ), 6.86(d,J=8.6Hz,1H),6.56(d,J=8.3Hz,1H),6.43(d,J=3.1Hz,1H),4.52(s,2H),4.12(q,J =7.3Hz,2H),1.45(t,J=7.3Hz,3H).
Embodiment 3
[0122] Example 3 Preparation of compound HYH2
[0123] It was prepared in the same manner as in Example 1 except that 2-benzyloxyphenylboronic acid was substituted for 2-benzyloxy-5-fluorophenylboronic acid pinacol ester.
[0124]
[0125] 1 H NMR(300MHz, CDCl 3 )δ7.61(d,J=8.4Hz,1H), 7.55(d,J=1.2Hz,1H), 7.25(dd,J=8.0,4.5Hz,2H), 7.14(d,J=3.1Hz, 1H), 7.06(dd,J=7.1,1.6Hz,1H), 6.99(dd,J=7.1,5.7Hz,2H), 6.91(dd,J=7.9,1.4Hz,1H), 6.50(dd,J =9.6,5.8Hz,2H),6.42-6.30(m,1H),4.54(s,1H),4.16(dd,J=14.5,7.2Hz,2H),1.48(t,J=7.3Hz,3H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com