Method for inactivating virus in source plasma of blood product
A blood product and virus inactivation technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and medical raw materials derived from mammals, etc., can solve the problems that the absolute safety of raw materials cannot be completely guaranteed, and achieve Quick and effective removal, easy to use, and the effect of reducing the chance of secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
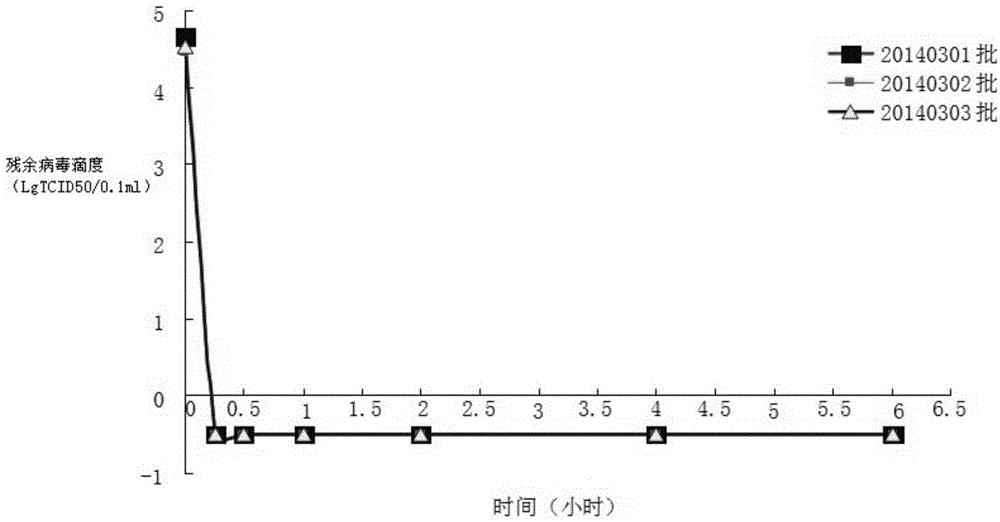
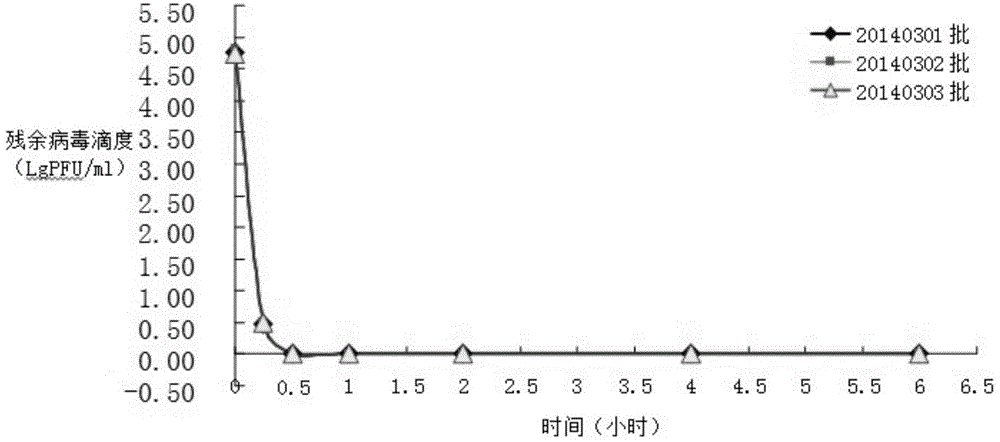
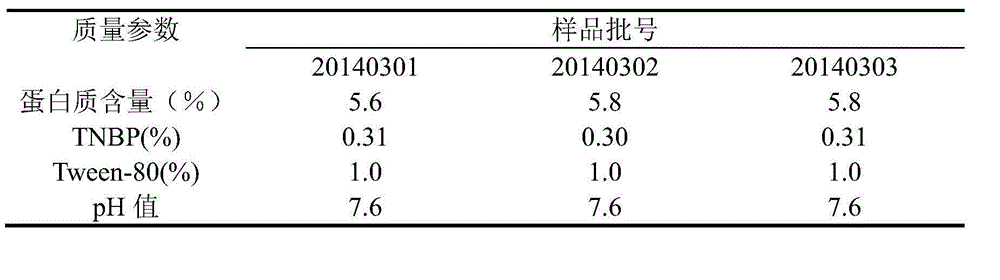
[0029] Multiple pooled human plasma was treated with virus using organic solvent and detergent mixture.
[0030] (1) plasma melting
[0031] Received 1,668 servings of plasma (net weight: 1,002.34 kg) from the cold storage that complied with the provisions of "human plasma for blood product production" in the third part of the "Chinese Pharmacopoeia" 2010 edition, and passed the re-inspection and met the quarantine period. The plasma was melted in a D-level clean area within. Use natural melting method or water bath melting method. The water temperature of the water bath melting method should be controlled below 37°C.
[0033] The plasma is merged, and the plasma is merged in a D-level clean area. Before the merger, the plasma bag should be disinfected, that is, soaked in 75% alcohol for more than three minutes, and the outer alcohol of the plasma bag can be wiped with clean gauze before merging. When merging plasma, attention should be paid to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com