Radical benzene sulfonamide ethyl sulfuryl hydroxyethyl sulfate aniline compound and preparation method thereof
A technology of ethylsulfone-based hydroxyethylsulfate and hydroxyethylsulfate, applied in the fields of sulfonamide preparation, chemical instruments and methods, organic chemistry, etc., can solve the problem of light fastness and soaping fastness The rubbing fastness is only 1-2 grades, which cannot meet the requirements of users and environmental protection, and the absolute color fixation rate is low, so as to achieve the effects of excellent fastness, bright color and good environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
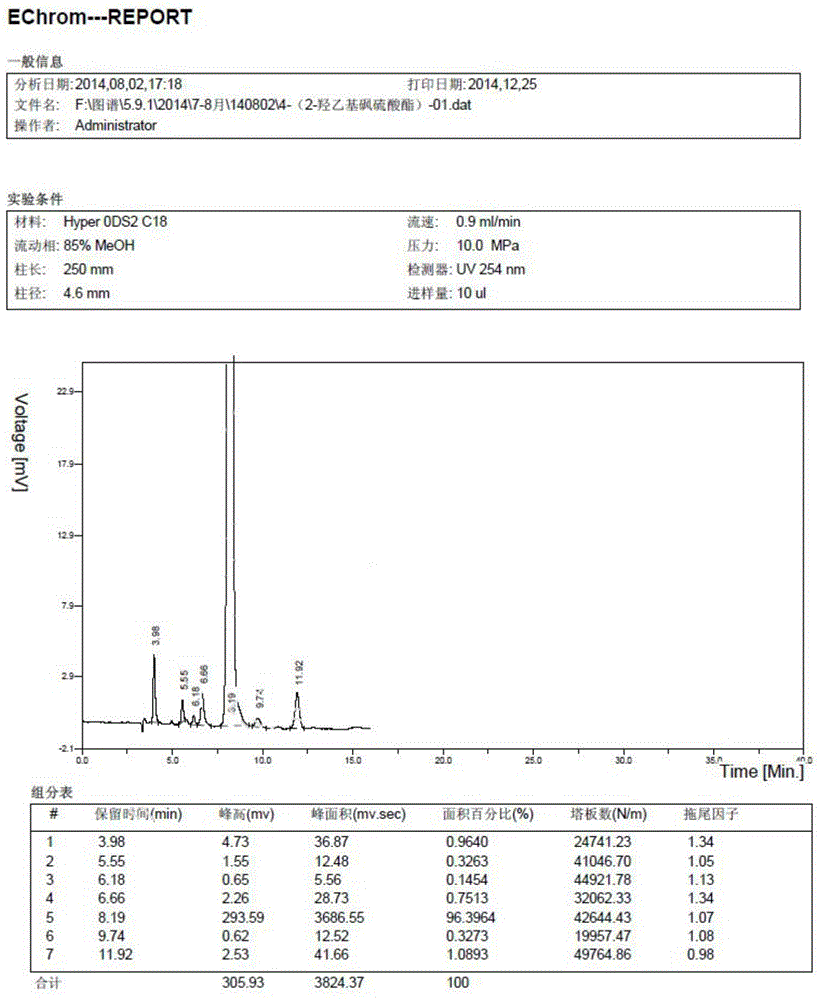
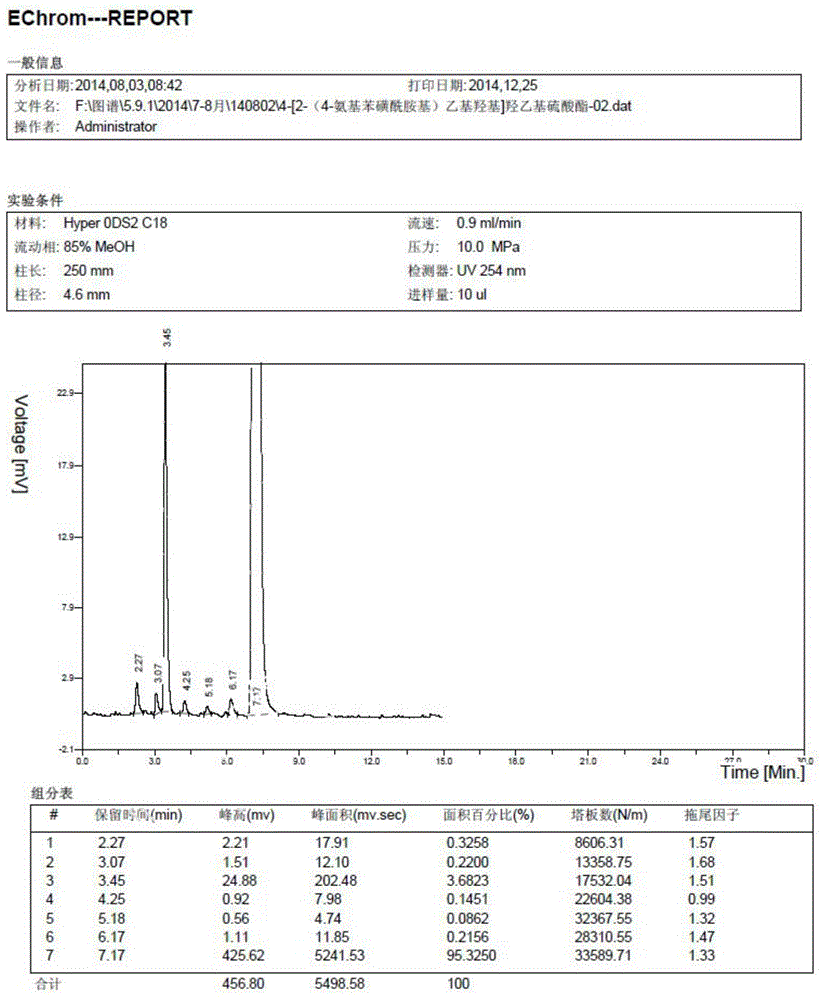
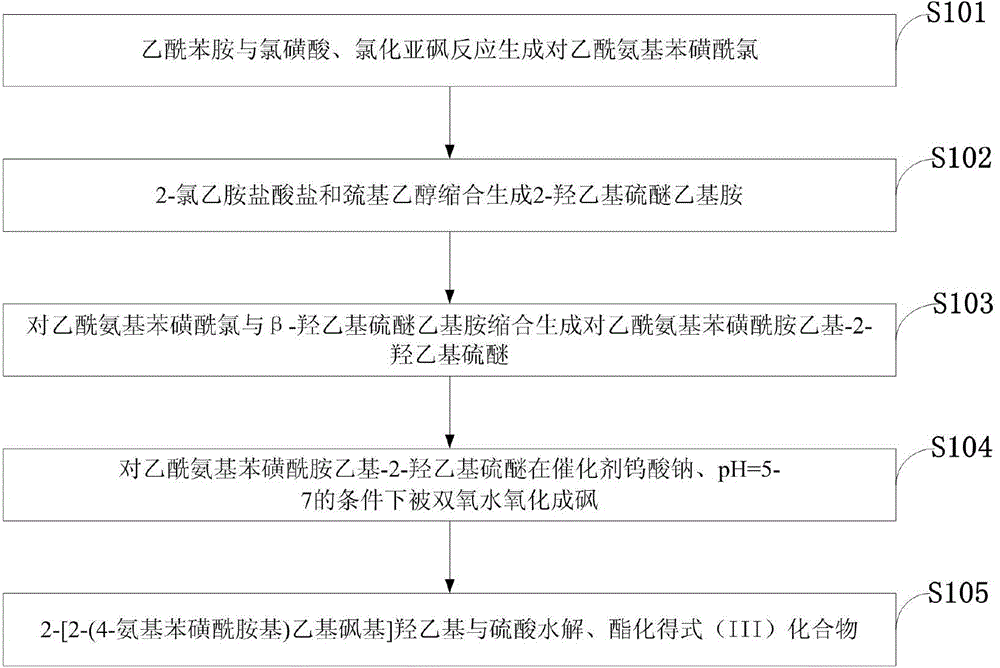
[0038] Such as image 3 Shown, the preparation method of the benzenesulfonamide ethyl sulfone base hydroxyethyl sulfate aniline compound of the embodiment of the present invention comprises:
[0039] S101: Acetanilide reacts with chlorosulfonic acid and thionyl chloride to generate p-acetamidobenzenesulfonyl chloride;
[0040] S102: Condensation of 2-chloroethylamine hydrochloride and mercaptoethanol to generate 2-hydroxyethyl sulfide ethylamine;
[0041] S103: Condensation of p-acetamidobenzenesulfonyl chloride and β-hydroxyethyl sulfide ethylamine to generate p-acetamidobenzenesulfonamide ethyl-2-hydroxyethyl sulfide;
[0042] S104: P-acetamidobenzenesulfonamide ethyl-2-hydroxyethyl sulfide is oxidized to sulfone by hydrogen peroxide under the conditions of catalyst sodium tungstate and pH=5-7;
[0043] S105: Hydrolysis and esterification of 2-[2-(4-aminobenzenesulfonamido)ethylsulfone]hydroxyethyl with sulfuric acid to obtain the compound of formula (III).
Embodiment 1
[0045] 1. Sulfonation reaction
[0046] Add 135 parts of acetanilide to 1154 parts of chlorosulfonic acid and control the temperature at 20-30°C. After the addition, slowly raise the temperature to 50°C for 2 hours, add 236.8 parts of thionyl chloride dropwise for 2 hours, cool down to 25°C and add ice water Diluted and filtered to obtain 513 parts (40.9%) of p-acetaminobenzenesulfonyl chloride.
[0047] 2. Condensation of mercaptoethanol
[0048] Add 119 parts of 2-chloroethylamine hydrochloride to 78 parts of mercaptoethanol solution under stirring, while adjusting the pH to 4.5 with baking soda, react at 45°C for 4 hours to generate 115.3 parts of 2-hydroxyethyl sulfide ethyl base amine.
[0049] 3. Condensation of acetaminobenzenesulfonyl chloride
[0050] After the end point is reached, the mercaptoethanol condensation liquid is cooled to 0°C, and 513 parts (40.9%) of the tide product p-acetaminobenzenesulfonyl chloride is slowly added, after the addition is completed,...
Embodiment 2
[0056] 1. Sulfonation reaction
[0057] Add 135 parts of acetanilide to 1154 parts of chlorosulfonic acid and control the temperature at 20-30°C. After the addition, slowly raise the temperature to 62°C for 2 hours, add 240 parts of thionyl chloride dropwise for 2 hours, cool down to 25°C and add ice water After dilution and filtration, 524 parts (41.2%) of p-acetaminobenzenesulfonyl chloride were obtained.
[0058] 2. Condensation of mercaptoethanol
[0059] Add 118.5 parts of 2-chloroethylamine hydrochloride to 78 parts of mercaptoethanol solution under stirring, and at the same time adjust pH=5 with baking soda, and react at 52°C for 4 hours to generate 116.5 parts of 2-hydroxyethyl sulfide ethyl base amine.
[0060] 3. Condensation of acetaminobenzenesulfonyl chloride
[0061] After the end point is reached, the mercaptoethanol condensation liquid is cooled to 0°C, and 524 parts (41.2%) of the tide product p-acetaminobenzenesulfonyl chloride is slowly added. After the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com